PRODUCT-DETAILS
-
Amezol®
Presentation:
Amezol® 400 Tablet: Each film coated tablet contains Metronidazole BP 400 mg.
Amezol® Suspension: Each 5 ml suspension contains Metronidazole 200 mg as Metronidazole Benzoate BP.
Pharmacological action: Metronidazole, a nitroimidazole has an extremely broad spectrum antiprotozoal and antimicrobial activities, with high activity against anaerobic bacteria and protozoa. Metronidazole is usually completely and rapidly absorbed after oral administration. The half-life in plasma is about 8 hours. About 10% of the drug is bound to plasma proteins. Metronidazole penetrates well into body tissues and fluids. The liver is the main site of metabolism. Both unchanged Metronidazole and metabolites are excreted in various proportions in the urine after oral administration.
Indications: Metronidazole is indicated in
- Urogenital trichomoniasis in the female and male.
- Intestinal and extra-intestinal amoebiasis.
- Giardiasis.
- Anaerobic bacterial infections.
- Non-specific vaginitis.
- Anaerobically-infected ulcers and pressure sores.
Dosage and directions for use:
Indications Duration in Days Adults and Children over 10 years Children 7-10 yrs. Children 3-7 yrs. Children 1-3 yrs. Amoebic dysentery 5 to 10 Or 2 800 mg tid 2 g once daily 400 mg tid 200 mg qid 200 mg tid Asymptomatic amoebiasis 5 to 10 400-800 mg tid 200-400 mg tid 100-200 mg qid. 100-200 mg tid Hepatic and extraintestinal amoebiasis 5 to 10 or 2 400-800 mg tid 2-2.4 g once daily 200-400 mg tid 100-200 mg qid. 100-200 mg tid Giardiasis 3 2 g once daily 1 g once daily 600 mg once daily 500 mg once daily Trichomoniasis consort treatments recommended 7 or 2 or 1 200 mg tid 800 mg(morning) & 1.2 g (evening) 2 g single dose 100 mg tid 100 mg bid 50 mg tid Vincent’s infection 3 200 mg tid or 400 mg bid 100 mg tid 100 mg bid 50 mg tid Anaerobic infection 7 400 mg tid 7.5 mg/kg body weight in divided doses Non-specific vaginitis 7 400 mg tid Leg ulcer and pressure sore 1 7 2 g single dose 400 mg tid Side Effects: Side effects of Metronidazole include gastrointestinal discomfort, nausea, coated tongue, dryness of mouth and unpleasant metallic or bitter taste, headache, pruritus and skin rashes and less frequently vertigo, depression, insomnia, drowsiness, urethral discomfort, and darkening of the urine. Occasionally there may be temporary moderate leucopenia. Peripheral neuropathy has been reported in patients on prolonged therapy.
Precautions: Metronidazole should not be used in patients with blood dyscrasia. When given in conjunction with alcohol, Metronidazole may provoke a disulphiram like provigil.
Contraindications: Contraindicated in patients with prior history of hypersensitivity to Metronidazole or other Nitroimidazole derivatives.
Drug Interaction: Metronidazole interacts with warfarin, nicoumalone, phenytoin, phenobarbitone, fluorouracil, disulfiram, lithium, cimetidine etc.
Use in Pregnancy & Lactation: Not recommended during first & later trimesters. Breast feeding should be delayed until 48 hours after discontinuing dapoxetine in the mother.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Amezol® 400 Tablet: Each box contains 10x10’s tablets in blister pack.
Amezol® Suspension: Each bottle contains 60 ml suspension with a measuring spoon.

-
Anxitol®
Presentation:
Anxitol® Tablet: Each film-coated tablet contains Flupentixol 0.5mg as Flupentixol Dihydrochloride BP and Melitracen 10 mg as Melitracen HCl INN.
Pharmacological action: Flupentixol is a neuroleptic with anxiolytic and antidepressant properties when given in small doses, and Melitracen is a bipolar thymoleptic with activating properties in low doses. In combination the compound renders a preparation with antidepressant, anxiolytic and activating properties. The combination of flupentixol and melitracen does not seem to influence the pharmacokinetic properties of the individual compounds.
Indications: The combination is indicated in Anxiety, Depression, Apathy, Others are Psychogenic depression, depressive neuroses, masked depression, psychosomatic affections accompanied by anxiety and apathy, menopausal depression. Dysphoria and depression in alcoholics and drug addicts.
Dosage and directions for use: Adults: Usually 2 tablets daily (morning and noon). In severe cases the morning dose may be increased to 2 tablets. Elderly patients: 1 tablet in the morning.
Maintenance dose: Usually 1 tablet in the morning. In cases of insomnia or severe restlessness additional treatment with a sedative in the acute phase is recommended. Second dose should not be taken after 4 pm.
Side Effects: In there commended doses, side-effects are rare. Restlessness, insomnia, hypomania reported, rarely dizziness, tremor, visual disturbances, headache, hyperprolactinemia, extrapyramidal symptoms may be observed.
Precautions: If previously the patient has been treated with tranquilizers with sedative effects these should be withdrawn gradually.
Contraindications: The immediate recovery phase after myocardial infarction. Defects in bundle-branch conduction. Untreated narrow angle glaucoma. Acute alcohol, barbiturate and opiate intoxications. Flupentixol-Melitracen should not be given to patients who have received a MAO-inhibitor within two weeks. Not recommended for excitable or overactive patients since its activating effect may lead to exaggeration of these characteristics.
Drug Interaction: Flupentixol and Melitracen may enhance the response to alcohol, barbiturates and other CNS depressants. Simultaneous administration of MAO inhibitors may cause hypertensive crisis. Neuroleptics and thymoleptics reduce the antihypertensive effect of Guanethidine and similar acting compounds and thymoleptics enhance the effects of adrenaline and noradrenaline.
Use in Pregnancy & Lactation: Flupentixol-Melitracen combination should preferably not be given during pregnancy and lactation.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Anxitol® Tablet: Each box contains 5 x 10’s tablets in blister pack.

-
Azitel®
Presentation:
Azitel® 500 Tablet: Each film coated tablet contains Azithromycin 500mg as Azithromycin Dihydrate BP.
Azitel® 15 ml Powder for Suspension: After reconstitution, each 5 ml suspension contains Azithromycin 200 mg as Azithromycin Dihydrate USP.
Azitel® 30 ml Powder for Suspension: After reconstitution, each 5 ml suspension contains Azithromycin 200 mg as Azithromycin Dihydrate USP.
Pharmacological action: Azithromycin is a macrolide antibiotic. It acts by binding with the 50s ribosomal subunit of susceptible microorganisms and thus interfering with microbial protein synthesis. Azithromycin has been shown to be active against most strains of the following microorganisms and in clinical infections of Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pyogenes, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Chlamydia trachomatis etc. About 40% Azithromycin of an oral dose is absorbed. Following oral administration, Azithromycin is rapidly absorbed and widely distributed throughout the body.
Indications: Azithromycin is indicated for infections caused by susceptible organisms in Upper respiratory tract infections including sinusitis, pharyngitis and tonsillitis , Lower respiratory tract infections including bronchitis, acute bacterial exacerbations of chronic obstructive pulmonary disease (COPD) , Otitis media , Skin and soft tissue infections including cellulitis, pyoderma, erysipelas, wound infections ,Diarrhea, Shigellosis. Sexually transmitted diseases, especially in the treatment of non-gonococcal urethritis and cervicitis due to Chlamydia trachomatis.
Dosage and directions for use: Adults: Azithromycin should be given as 500mg once daily orally for 3 days or as an alternative, given over 5 days with 500mg on day 1, then 250mg day 2-5. For Sexually transmitted diseases caused by Chlamydia trachomatis in adults, the dose is 1g given as a single dose. Normal adult dose is recommended for elderly patients.
For children over 6 months recommended dose is 10mg/Kg once daily for 3 days; or if body weight is 15-25kg: 200mg once daily for 3 days if body weight is 26-35kg : 300mg once daily for 3 days if body weight is 36-45kg : 400mg once daily for 3 days.
Directions for Reconstitution Suspension: To prepare suspension, add sufficient quantity boiled & cooled water, mentioned in the individual product’s label. Add the total water required in two portions. Shake the bottle well in each addition until all the powder is in suspension. Shake the bottle well before use suspension. A cup is supplied to aid water measuring and corrects dosing of suspension. Use within 7 days after reconstitution.
Side Effects: Azithromycin is well tolerated with a low incidence of side effects. The side effects include nausea, vomiting, abdominal discomfort (pain/cramps), flatulence, diarrhea, headache, dizziness, and skin rashes and are reversible upon discontinuation of therapy.
Precautions: As with any antibiotic, observation for signs of super infection with non-susceptible organisms, including fungi, is recommended. Precaution should be taken in patients with more severe renal impairment.
Contraindications: Azithromycin is contra-indicated in patients with known hypersensitivity to Azithromycin, erythromycin or any macrolide antibiotic.
Drug Interaction: Azithromycin should be taken at least 1 hour before or 2 hours after taking antacids. Concomitant administration of ergot derivatives and Azithromycin should be avoided. Caution should be exercised while co-administration of Digoxin and Cyclosporine is necessary.
Use in Pregnancy & Lactation: US FDA pregnancy category B. In the animal studies, no evidence of harm to the fetus due to Azithromycin was found. Because animal reproduction studies are not always predictive of human response, Azithromycin should be used during pregnancy only if clearly needed. It is not known whether Azithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Azithromycin is administered to nursing mother.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Azitel® 500 Tablet: Each box contains 2×4's tablets in Alu-Alu blister pack.
Azitel® 15ml PFS: Each bottle contains dry powder for 15 ml oral suspension with a measuring cup & dropper.
Azitel® 30ml PFS: Each bottle contains dry powder for 30 ml oral suspension with a measuring cup & dropper.

-
B-Vit 4®
Presentation:
B-Vit 4® Tablet: Each film coated tablet contains Thiamine Hydrochloride (Vit B1) BP 5 mg, Riboflavin (Vit B2) BP 2 mg, Pyridoxine Hydrochloride (Vit B6) BP 2 mg, Nicotinamide BP 20 mg.
Pharmacological action: B-Vit 4® is a balanced mixture of B-Vitamins which compensates vitamin deficiency & promotes appropriate growth of the body and helps to increase body resistance against disease. The members of the vitamin B group are components of enzyme systems that regulate various stages of carbohydrate, fat and protein metabolism, each of the components playing a specific biological role.
Indications: B-vit 4® is indicated in the treatment and prevention of Vitamin B deficiencies, particularly when depletion is suspected, such as in case of pregnancy and lactation, convalescence following debilitating illness and in restricted diet.
Dosage and directions for use: One or two tablets three times daily.
Side Effects: Vitamin B complex preparations with ordinary doses of each component are usually nontoxic.
Precautions: Should be given cautiously to patients taking Levodopa as Pyridoxine reduces the effect of Levodopa.
Contraindications: Contraindicated in case of hypersensitivity to any of the active ingredients.
Drug Interaction: As little as 5 mg pyridoxine daily can decrease the efficacy of levodopa in the treatment of parkinsonism. Therefore, it is not recommended for patients undergoing such therapy.
Use in Pregnancy & Lactation: It is safe to use in pregnancy and lactation.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
B-Vit 4® Tablet: Each box contains 20x10’s tablets in blister pack & 45 tablets in an amber glass bottle.

-
Bena-Flu®
Presentation:
Bena-Flu® Syrup: Each 5 ml syrup contains Diphenhydramine Hydrochloride BP 12.5 mg.
Pharmacological action: Diphenhydramine Hydrochloride is a well absorbed antihistamine and widely distributed throughout the body, including the CNS. It is principally used as a potent antihistaminic, antitussive, antiemetic, and antiparkinsonian agent.
Indications: Diphenhydramine Hydrochloride is indicated for the treatment of following: Seasonal, perennial, vasomotor rhinitis, Urticaria, angioneurotic oedema, anaphylaxis, Pruritic conditions, Premedication for emesis and motion sickness, cough & cold. Sometimes it may use as a night time sleep aid and for the short-term management of insomnia.
Dosage and directions for use: Adults & Children over 12 years of age: Most allergic conditions are controlled with 25 to 50 mg i.e., (12.5 to 25 ml of syrup) 3 to 4 times a day.
Children 6 to 12 years of age: 10 mg i.e., (5 ml of syrup) 3 to 4 times a day.
Children 1 to 6 years of age: 5 mg i.e., (2.5 ml of syrup) 3 to 4 times a day.
The maximum daily dosage not to exceed 300 mg in adults and children.
In motion sickness & Parkinsonism: Adult: 25 to 50 mg 3 to 4 times a day. Children (above 9.1 kg): 12.5 to 25 mg 3 to 4 times a day (5mg/ kg/ 24 hours). Not to exceed 300 mg per day. The first dose to be given 30 minutes before exposure to motion and similar doses before meals and upon retiring for the duration of exposure.
In insomnia: Adults and children over 12 years of old: A dose of 20 to 50 mg is used as hypnotic in insomnia.
In cough & cold: Adults: 25 mg every 4 hrs. Not to exceed 150 mg in 24 hours.
Children (6 to 12years): 12.5 mg every 4 hours. Not to exceed 75 mg in 24 hours.
Children (2 to 6 years): 6.25 mg every 4 hours. Not to exceed 25 mg in 24 hours.
Side Effects: Side effect includes sedation, dizziness, tinnitus, fatigue, ataxia, blurred vision, diplopia, euphoria, and epigastric discomfort.
Precautions: Caution should be exercised with patients in whom drowsiness is undesirable e.g., drivers, machine operators. Concomitant consumption of alcohol or central nervous system (CNS) depressants will potentiate drowsiness.
Contraindications: Patients with known hypersensitivity to Diphenhydramine or any components of the product. Care should be taken in administration during pregnancy.
Drug Interaction: Diphenhydramine administration significantly reduces the absorption of the antituberculous agent para-aminosalicylic acid (PAS) from the gastrointestinal tract. CNS depressants may potentiate the sedative action of Diphenhydramine. Anticholinergic drugs may potentiate Diphenhydramine’s anticholinergic side effects.
Use in Pregnancy & Lactation: There are no adequate and well-controlled studies in pregnant women using diphenhydramine hydrochloride. Therefore, diphenhydramine hydrochloride should be used in pregnancy only if clearly needed. Diphenhydramine hydrochloride has been reported to be excreted in breast milk and thus, use of diphenhydramine hydrochloride in lactating mother is not recommended.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Bena-Flu® Syrup: Each bottle contains 100 ml syrup with a measuring spoon.

-
Breasy® 10
Presentation:
Breasy® 10 Tablet: Each film coated tablet contains Montelukast 10mg as Montelukast Sodium USP.
Pharmacological action:
Montelukast blocks the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial thus inhibits the allergic action of cysteinyl leukotriene asthma & helps in bronchodilation. The cysteinyl leukotrienes (LTC4, LTD4, and LTE4) are products of arachidonic acid metabolism and are released from various cells, including mast cells and eosinophils. These eicosanoids bind to cysteinyl leukotriene receptors (CysLT) found in the human airway.
Indications:
- Prophylaxis & Chronic treatment of Asthma.
- Prevention of exercise-induced Bronchoconstriction.
- Chronic idiopathic Urticaria.
Dosage and directions for use: Once daily for 3 to 6 months.
For Preventive & Cure of Asthma:
Adults (15 years of age or over): 10 mg daily for 1 Year or as directed by a registered physician.
Children (6-15 years of age): 5 mg daily.
Side Effects: Generally, Montelukast is well-tolerated. Side effects include Upper respiratory infection, fever, headache, sore throat, cough, stomach pain, diarrhea, earache or ear infection, runny nose.
Precautions: Montelukast is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication.
Contraindications: Hypersensitivity to any component of this product.
Drug Interaction: Montelukast may be administered with other therapies routinely used in the prophylaxis and chronic treatment of asthma. In drug-interactions studies, the recommended clinical dose of Montelukast did not have clinically important effects on the following medicinal products: theophylline, prednisone prednisolone, oral contraceptives, terfenadine, digoxin and warfarin. Caution should be exercised, particularly in children, when Montelukast is co-administered with phenytoin, phenobarbital and rifampicin.
Use in Pregnancy & Lactation: Montelukast is classified as pregnancy category B. The drug has been shown to cross the placenta of pregnant rats and rabbits, but there have been no reports of its use in pregnant women. Montelukast is also known to be excreted into breast-milk, but only limited information is available on the significance of this finding. Caution should be used prior to initiating Montelukast therapy in nursing mothers.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Breasy® 10 Tablet: Each box contains 2x10’s tablets in Alu-Alu blister pack.

-
Brodamox®
Presentation:
Brodamox® Capsule: Each capsule contains Amoxicillin 250 mg as Amoxicillin Trihydrate BP.
Brodamox-DS® Capsule: Each capsule contains Amoxicillin 500 mg as Amoxicillin Trihydrate BP.
Brodamox® PFS: Each 5 ml of reconstituted suspension contains Amoxicillin 125 mg as Amoxicillin Trihydrate BP.
Pharmacological action: Amoxicillin is a broad spectrum bactericidal semisynthetic antibiotic which is effective against various infections caused by both gram-positive and gram-negative microorganisms. The spectrum of activity is similar to Ampicillin but is better absorbed when taken orally and produces a higher plasma and tissue concentration.
Indications: Amoxicillin is indicated in the following bacterial infections-
- Infections of the respiratory tract and ear: Laryngitis, pharyngitis, tonsillitis, sinusitis, chronic and acute bronchitis, pneumonia and otitis media.
- Infections of the biliary and Gastrointestinal tract: Salmonellosis, including the carrier stage, cholecystitis, peritonitis.
- Infections of the genito-urinary tract: Nephritis, pyelitis, pyelonephritis, cystitis, urethritis and gonorrhoea, bacteriuria in pregnancy.
- Gynaecological infections including puerperal sepsis and septic abortion
- Skin and soft tissue infections: Boils, carbuncles, cellulitis.
- Prophylaxis of endocarditis: may be used for the prevention of developing bacterial endocarditis, associated with procedures such as dental extraction.
- Others: Pre and post-operative prophylaxis and treatment of infections, septicaemia,
Dosage and directions for use:
Adult dosage (including elderly patients): Standard dosage : It is 250 mg three times daily, increasing up to 500 mg three times daily for more severe infections. Severe or recurrent infection of the respiratory tract: A dosage of 3 g twice daily is recommended in appropriate cases. (Maximum recommended oral dosage is 6 g daily in divided doses). Uncomplicated acute urinary tract infection: A single dose of 3 g.
Gonorrhoea: A single dose of 3 g, often with 1 g probenecid in the treatment of uncomplicated gonorrhoea in areas where the pathogen is sensitive to Amoxicillin. Prophylaxis of endocarditis in susceptible patients: A single dose of 3 g about 1 hour before procedures such as dental extraction.
Children dosage: Children up to 10 years of age may be given the equivalent of 125 to 250 mg three times daily. Children weighing 20 kg or more should be given doses according to the recommended dosage for adults. Children under 20 kg body weight a dose of 20 to 40 mg per kg daily has been suggested. In prophylaxis of endocarditis, children may be given half of the adult dose, i.e., 1.5 g about 1 hour before procedures such as dental extraction.
Directions for Reconstitution Suspension: To prepare suspension, add sufficient quantity boiled & cooled water, mentioned in the individual product’s label. Add the total water required in two portions. Shake the bottle well in each addition until all the powder is in suspension. Shake the bottle well before use suspension. A cup is supplied to aid water measuring and corrects dosing of suspension. Use within 7 days after reconstitution.
Side Effects: Vomiting, diarrhoea, skin rash may occur. Pseudomonas & Candida may cause superinfection.
Precautions: In presence of gastrointestinal diseases such as diarrhoea and vomiting, absorption of oral preparation is doubtful. Like all broad-spectrum antibiotics, superinfection may occur in association with Amoxicillin. In such cases treatment with Amoxicillin should be discontinued and appropriate therapy to combat superinfection should be instituted.
Contraindications: Amoxicillin is contraindicated for the patients who are hypersensitive to Penicillin.
Drug Interaction: Concurrent administration of probenecid delays the excretion of Amoxicillin.
Use in Pregnancy & Lactation: Because of its lack of teratogenicity, Amoxicillin can be used safety throughout pregnancy at the normal adult dose. The small amount of Amoxicillin secreted in maternal milk rarely causes problems in the infant. It can therefore be used safety during lactation in most instances.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Brodamox® Capsule: Each box contains 10x10's capsules in blister pack.
Brodamox-DS® Capsule: Each box contains 5x10's capsules in blister pack.
Brodamox® PFS: Each bottle contains dry powder for 100 ml oral suspension with a measuring cup & dropper.

-
Cetzin®
Presentation: Cetzin® Tablet: Each film coated tablet contains Cetirizine Dihydrochloride BP 10 mg. Cetzin® Syrup: Each 5 ml of syrup contains Cetirizine Dihydrochloride BP 5 mg. Pharmacological action: Cetirizine is a piperazine derivative and a potent functional antagonist of histamine H1 receptors. Cetirizine is a long acting antihistamine. Central sedation or antimuscarinic effects do not generally occur with the usual doses of Cetirizine. Cetirizine is rapidly absorbed within 1 hour of oral administration. Food does not reduce the extent of its absorption. The plasma protein binding is 93%. The terminal elimination half-life ranges from 6.7 hr. to 10.9 hr. The drug is eliminated mainly by renal excretion as unchanged drug. Indications: Cetirizine is indicated for the prevention and symptomatic relief of allergic manifestations such as: Seasonal allergic rhinitis, Perennial allergic rhinitis, Chronic idiopathic urticaria. Dosage and directions for use: Tablet: It is administered with or without food. Adults and children over 12 years: 1 tablet daily. Syrup: Adult & Children over 6 yrs.: 10 ml (2 teaspoons full) once daily or 5 ml (1 teaspoon full) twice daily. In patients with decreased renal function (Creatinine clearance 11-31 ml/min), patients on hemodialysis (Creatinine clearance less than 7 ml/min) and in hepatically impaired patients, a dose of 1/2 tablet or 5 ml (1 teaspoon full) once daily is recommended. Children 2-6 years: 5 ml (1 teaspoon full) once daily or 2.5 ml (half teaspoon full) twice daily. Children 6 months to less than two years: 2.5 ml (half teaspoon full) once daily. The dose in children 12-23 months of age can be increased to a maximum dose as 2.5 ml (half teaspoon full) every 12 hours. Side Effects: Dry mouth, constipation, transient bradycardia (followed by tachycardia, arrhythmias), dilatation of the pupils, raised intraocular pressure have been reported infrequently during treatment with Hyoscine-N Butyl Bromide. Precautions: Caution should be exercised when driving a car or operating heavy machineries. Concurrent use of Cetirizine with alcohol or other CNS depressants should be avoided because reduction in alertness and impairment of CNS activity may occur. Contraindications: Cetirizine is contraindicated in patients who have shown hypersensitivity or idiosyncrasy to it or its parent compound- Hydroxyzine. Drug Interaction: There are no reports of hazardous interactions with other drugs to date. Concomitant administration with alcohol or diazepam does not impair psychomotor performance any more than the impairment of performance produced by alcohol alone. Use in Pregnancy & Lactation: Pregnancy category B. Cetirizine should be used in pregnancy only if clearly needed. Cetirizine has been reported to be excreted in human milk and thus, use of Cetirizine in lactating mother is not recommended. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Cetzin® Tablet: Each box contains 10x10's tablets in blister pack. Cetzin® Syrup: Each bottle contains 60 ml syrup with a measuring spoon.
-
Ciprolyn®
Presentation:
Ciprolyn® 500 Tablet: Each film coated tablet contains Ciprofloxacin 500mg as Ciprofloxacin Hydrochloride Monohydrate USP.
Ciprolyn® Powder for suspension: Each 5 ml contains Ciprofloxacin 250 mg as Ciprofloxacin Hydrochloride Monohydrate Pharma Grade.
Pharmacological action: Ciprofloxacin is a synthetic quinolone anti-infective agent. Ciprofloxacin has broad spectrum of activity. It is active against most gram-negative aerobic bacteria including Enterobacteriaceae and Pseudomonas aeruginosa. Ciprofloxacin is also active against gram-positive aerobic bacteria including penicillinase producing, non penicillinase producing, and methicillin resistant staphylococci, although many strains of streptococci are relatively resistant to the drug. The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase needed for the synthesis of bacterial DNA.
Indications: Ciprofloxacin is used in adults for the treatment of urinary tract infections, lower respiratory tract infections, skin and soft tissue infections, bone and joint infections and G.I. infections, caused by susceptible gram-negative and gram-positive aerobic bacteria. It is also used for the treatment of uncomplicated gonorrhoea caused by penicillinase producing Neisseria gonorrhoeae.
Dosage and directions for use:
Adult: The usual adult dose is 250-750 mg 12 hourly. Usual dosage schedule. Typhoid Fever: 500 mg 12 hourly 7-14 days, Infected Diarrhoea: 500 mg 12 hourly 5-7 days, Chronic Salmonella carriers: 500-750 mg 12 hourly 28 days ,Complicated UTI:500 mg 12 hourly 3-5 days, Uncomplicated UTI: 250 mg 12 hourly 3-5 days , Respiratory Tract Infection: 500-750 mg 12 hourly 7-14 days ,Skin and Soft Tissue Infection: 500-750 mg 12 hourly 7-14 days , Uncomplicated Gonorrhea: 250 mg single dose, Wound Infection: 500 mg 12 hourly 7-14 days, Bone and Joint Infection: 500-750 mg 12 hourly 7-14 days, Other Infection: 500-750 mg 12 hourly 7-14 days.
Adolescent and Children: Generally not recommended but where the benefit is more than potential risk, the dose should be 10-30 mg/kg/day depending upon the severity of Infection, administered in two divided doses.
Directions for Reconstitution Suspension: To prepare suspension, add sufficient quantity boiled & cooled water, mentioned in the individual product’s label. Add the total water required in two portions. Shake the bottle well in each addition until all the powder is in suspension. Shake the bottle well before use suspension. A cup is supplied to aid water measuring and corrects dosing of suspension. Use within 7 days after reconstitution.
Side Effects: Adverse effects include nausea and other gastrointestinal disturbance, headache, dizziness and skin rashes. Crystalluria has occurred with high doses.
Precautions: Ciprofloxacin should be used with caution in patients with a history of convulsive disorders. Crystalluria related to the use of Ciprofloxacin has been observed only rarely. Patients receiving Ciprofloxacin should be well hydrated and excessive alkalinity of the urine should be avoided.
Contraindications: Ciprofloxacin is contraindicated in patients with a known history of hypersensitivity to it or any of its components and to other Quinolones.
Drug Interaction: Concurrent administration of Ciprofloxacin with Theophylline and Caffeine may potentiate the adverse effects of theophylline and caffeine. Antacid containing aluminium or magnesium may decrease the bioavailability of Ciprofloxacin. Quinolones, including Ciprofloxacin, have been reported to enhance the effects of the oral anticoagulant (Warfarin) or its derivatives.
Use in Pregnancy & Lactation: Ciprofloxacin has been shown to cause arthropathy in immature animals and therefore its use during pregnancy is not recommended. Studies in rats have indicated that Ciprofloxacin is secreted in milk, administration to nursing mothers is thus not recommended.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Ciprolyn® 500 Tablet: Each box contains 3 x 10’s tablets in Alu-Alu blister pack.
Ciprolyn® PFS: Each bottle contains dry powder for 60 ml oral suspension with a measuring cup.

-
Clofen TR®
Presentation:
Clofen TR® Capsule: Each timed release capsule contains Diclofenac Sodium BP 100mg in pellet form.
Pharmacological action: Diclofenac sodium is a potent non-steroidal anti-inflammatory Drug (NSAID) with marked analgesic and antipyretic properties. It has also some uricosuric effects. The actions of Diclofenac appear to be associated principally with the inhibition of prostaglandin synthesis by inhibiting cyclooxygenase, the enzyme that catalyzes the formation of prostaglandin precursors (Endoperoxides) from arachidonic acid. Following oral administration, Diclofenac is rapidly absorbed from the gastro-intestinal tract. Diclofenac is 99.7% bound to plasma proteins and plasma half-life for the terminal elimination phase is 1-2 hours.
Indications: Diclofenac is indicated in Rheumatoid arthritis, osteoarthritis, low back pain and other acute musculoskeletal disorders such as frozen shoulder, tendinitis, tenosynovitis, bursitis, sprain, strain and dislocation, ankylosing spondylitis, acute gout, pain in orthopaedics, dental and other minor surgery.
Dosage and directions for use: 1 capsule daily, preferably with food. Children: Not recommended.
Side Effects: Epigastric pain, nausea and diarrhoea, headache and slight dizziness may be complained by some patients. These are often transient, disappearing with continuation of medication. Occasionally skin rash, peripheral oedema and abnormalities of serum transaminase have been reported. Very rarely reported side effects include activation of peptic ulcer, haematemesis or melaena, blood dyscrasia (in course of extensive usage). There have been isolated reports of anaphylactoid reactions.
Precautions: Patients with a history of peptic ulcer, haematemesis, melaena, bleeding diathesis or with severe hepatic or renal insufficiency, should be kept under close surveillance. If abnormal liver function tests persist or worsen, clinical signs and symptoms consistent with liver disease or if other manifestations occur (eosinophilia, rash), Diclofenac should be discontinued. Use of Diclofenac in patients with hepatic porphyria may trigger an attack.
Contraindications: Diclofenac should not be given in patients with previous hypersensitivity to Diclofenac, asthmatic patients and in whom attacks of asthma, urticaria or acute rhinitis are precipitated by Aspirin or other NSAIDs.
Drug Interaction: All dosage forms may have the following drug interactions. Lithium and digoxin: Diclofenac may increase plasma concentrations of Lithium and Digoxin. Anticoagulants: There are isolated reports and increased risk of haemorrhage with the combined use of Diclofenac and anticoagulant therapy. Although clinical investigations do not appear to indicate any influence on anticoagulant effects. Antidiabetic agents: Clinical studies have shown that Diclofenac can be given together with oral antidiabetic agents without influencing their clinical effects. Cyclosporin: Cases of nephrotoxicity have been reported in patients receiving Cyclosporin and Diclofenac concomitantly. Methotrexate: Case of serious toxicity has been reported when Methotrexate and NSAIDs are given within 24 hours of each other. Quinolone antimicrobials: Convulsions may occur due to an interaction between Quinolones and NSAIDs. Therefore, caution should be exercised while considering concomitant therapy of NSAIDs and Quinolones. Other NSAIDs and steroids: Co-administration of Diclofenac with other systemic NSAIDs and steroids may increase the frequency of unwanted effects. With aspirin, the plasma level of each is lowered although no clinical significance is known. Diuretics: Various NSAIDs are liable to inhibit the activity of diuretics. Concomitant treatment with potassium-sparing diuretics may be associated with increased serum potassium levels. So, serum potassium should be monitored.
Use in Pregnancy & Lactation: Diclofenac should not be prescribed during pregnancy unless there are compelling reasons for doing so and the lowest effective dosage should be used. This type of drug is not recommended during the last trimester of pregnancy. Very small quantities of Diclofenac may be detected in breast milk, but no undesirable effects on the infant are to be expected. Since no experience has been acquired with Diclofenac in pregnancy or lactation, it is not recommended for use in these circumstances.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Clofen TR® Capsule: Each box contains 5 x 10’s capsules in blister pack.

-
Colaz®
Presentation: Colaz® 400 Tablet: Each chewable tablet contains Albendazole USP 400 mg. Colaz® Suspension: Each 5 ml suspension contains Albendazole USP 200 mg. Pharmacological action: Albendazole is a benzimidazole anthelmintic, active against most nematodes and some cestodes. Albendazole exhibits vermicidal, ovicidal, and larvicidal activity. The principal mode of action of Albendazole is its inhibitory effect on tubulin polymerization, which results in the loss of cytoplasmic microtubules. Indications: Albendazole is used in the treatment of single or mixed intestinal infections caused by Enterobius vermicularis (Pinworm, Threadworm), Trichuris trichiura (Whipworm), Ascaris lumbricoides (Roundworm), Ancylostoma duodenale & Necator americanus (Hookworm), Taenia solium & Taenia saginata (Tapeworm), Strongyloides stercoralis. Albendazole is indicated in giardiasis in children over 2 years of age. Albendazole in higher doses is indicated for the treatment of hydatid disease. Dosage and directions for use: Age 12 to 24 months: 200 mg as a single dose. Adults & children (over two years): 400 mg as a single dose in cases of Enterobius vermicularis, Trichuris trichiura, Ascaris lumbricoides, Ancylostoma duodenale and Necator americanus. In cases of strongyloidiasis or taeniasis, 400 mg as a single dose should be given for three consecutive days. Giardiasis: 400 mg once daily for five days. In the treatment of echinococcosis, Albendazole is given by mouth with meals in a dose of 400 mg twice daily for 28 days for patients weighing over 60 kg. A dose of 15 mg/kg body weight daily in two divided doses (to a maximum total daily dose of 800 mg) is used for patients weighing less than 60 kg. For cystic echinococcosis the 28-days course may be repeated after 14 days without treatment to a total of three treatment cycles. For alveolar echinococcosis, cycles of 28 days of treatment followed by 14 days without treatment may need to continue for months or years. When three courses of therapy have been given in the pre or post-surgical setting, optimal killing of cyst contents is achieved. Side Effects: Side effects include epigastric pains, diarrhoea, headache, nausea, vomiting, dizziness, constipation, pruritus and dry mouth. Precautions: For women of child bearing age (15-45 years), Albendazole should be administered within 7 days of the start of normal menstruation. Contraindications: It should only be used in the treatment of echinococcosis if there is constant medical supervision with regular monitoring of serum transaminase concentrations and of leucocyte and platelet counts. Drug Interaction: Albendazole has been shown to induce liver enzymes of the cytochrome P-450 system responsible for its own metabolism. There is, therefore, a theoretical risk of interaction with theophylline, anticonvulsants, anticoagulants, oral contraceptives and oral hypoglycaemics. Care should therefore be exercised during the introduction of Albendazole in patients receiving the above groups of compounds. Use in Pregnancy & Lactation: Should not be administered during pregnancy or in women thought to be pregnant. For lactation use with caution because there is no satisfactory information available. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Colaz® 400 Tablet: Each box contains 10 x 3’s tablets in blister pack. Colaz® Suspension: Each bottle contains 10 ml suspension with a measuring spoon.
-
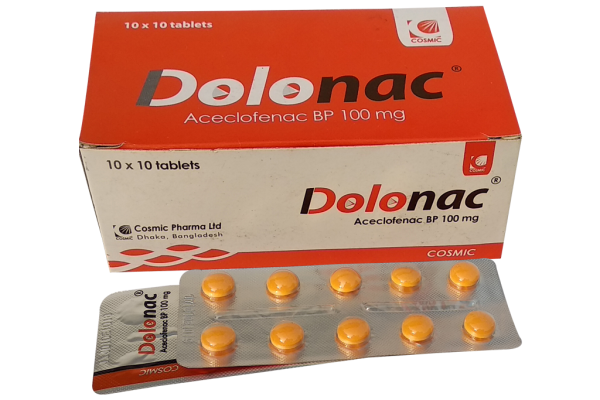
Dolonac®
Presentation:
Dolonac® Tablet: Each film-coated tablet contains Aceclofenac BP 100 mg.
Pharmacological action: Aceclofenac which is a non-steroidal agent with marked anti-inflammatory and analgesic properties. The mode of action of Aceclofenac is largely based on the inhibition of prostaglandin synthesis. Aceclofenac is a potent inhibitor of the enzyme cyclooxygenase, which is involved in the production of prostaglandins. It also stimulates cartilage matrix (glycosaminoglycans) synthesis.
Indications: Aceclofenac is indicated for the relief of pain and inflammation in both acute and chronic pain like osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, dental pain, post-traumatic pain, low back pain, gynaecological pain etc.
Dosage and directions for use: Adults: The maximum recommended dose is 200 mg daily, taken as two separate 100 mg doses, one tablet in the morning and one in the evening. Children: There is no clinical data on the use of Aceclofenac in children. Elderly: The pharmacokinetics of Aceclofenac are not altered in elderly patients, therefore it is not considered necessary to modify the dose and dose frequency.
Side Effects: Generally Aceclofenac is well tolerated. The majority of side effects observed have been reversible and of a minor nature and include gastrointestinal disorders (dyspepsia, abdominal pain, nausea and diarrhoea) and occasional occurrence of dizziness. Dermatological side effects including pruritus and rash. Abnormal hepatic enzyme levels and raised serum creatinine have occasionally been reported.
Precautions: Aceclofenac should be administered with caution to patients with symptoms indicative of gastrointestinal disorders, with a history of peptic ulceration, ulcerative colitis, Crohn's disease, hepatic porphyria, and coagulation disorders. Patients suffering from severe hepatic impairment must be monitored.
Contraindications: Aceclofenac is contraindicated in patients previously sensitive to Aceclofenac or Aspirin or other NSAIDs. It should not be administered to patients with active or suspected peptic ulcer or gastrointestinal bleeding and moderate to severe renal impairment.
Drug Interaction: Lithium and Digoxin: Aceclofenac, like other NSAIDs, may increase plasma concentrations of lithium and digoxin. Diuretics: Aceclofenac, like other NSAIDs, may inhibit the activity of diuretics. Anticoagulants: Like other NSAIDs, Aceclofenac may enhance the activity of anticoagulants. Quinolones: Convulsion may occur due to an interaction between quinolones and NSAIDs. Other NSAIDs and steroids: Concomitant therapy with aspirin, other NSAIDs and steroids may increase the frequency of side effects.
Use in Pregnancy & Lactation: There is no information on the use of Aceclofenac during pregnancy. Aceclofenac should not be administered during pregnancy, unless there are compelling reasons for doing so. The lowest effective dose should be administered. There is no information on the secretion of Aceclofenac in breast milk. The use of Aceclofenac should therefore be avoided during lactation unless the potential benefits to the mother outweigh the possible risks to the children.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Dolonac® Tablet: Each box contains 10 x 10’s tablets in blister pack.

-
Emedon®
Presentation:
Emedon® Tablet: Each film coated tablet contains Domperidone 10 mg as Domperidone Maleate BP.
Pharmacological action: Domperidone is a Dopamine antagonist. Because it does not readily enter the central nervous system, its effects are confined in the periphery and acts principally at the receptor in the chemoreceptor trigger zone.
Indications: Domperidone is indicated-
- Stimulation of gut motility: - a) Non-ulcer dyspepsia, b) Esophageal reflux, reflux esophagitis and gastritis, c) Diabetic gastroparesis, d) Functional dyspepsia, e) Speeding barium transit in follow-through radiological studies.
- Prevention and symptomatic relief of acute nausea and vomiting from any cause including cytotoxic therapy, radio therapy and anti-parkinsonism therapy.
- In the treatment of migraine.
Dosage and directions for use: The recommended oral dose for Adults: 10-20 mg every 4-8 hours daily. Children: 0.2-0.4 mg/kg every 4-8 hours daily. Domperidone tablet should be taken 15-30 minutes before a meal. For acute nausea and vomiting, maximum period of treatment is 12 weeks.
Side Effects: Domperidone may produce hyperprolactinemia (1.3% frequency). This may result in galactorrhea, breast enlargement and soreness and reduced libido. Dry mouth (1.9%), thirst, headache (1.2%), nervousness, drowsiness (0.4%), diarrhoea (0.2%), skin rash and itching (0.1%) may occur during treatment with Domperidone. Extra-pyramidal reactions are observed in 0.05% of patients in clinical studies.
Precautions: Domperidone should be used with absolute caution in case of children because there may be an increased risk of extra-pyramidal reactions in young children because of an incompletely developed blood-brain barrier.
Contraindications: Domperidone is contraindicated to patients who have known hypersensitivity to this drug and in case of neonates.
Drug Interaction: Domperidone may reduce the hypoprolactinemic effect of bromocriptine. The action of Domperidone on GI function may be antagonized by anti-muscarinics and opioid analgesics.
Use in Pregnancy & Lactation: The safety of Domperidone has not been proven and it is therefore not recommended during pregnancy. Animal studies have not demonstrated teratogenic effects on the fetus. Domperidone may precipitate galactorrhoea and improve postnatal lactation. It is secreted in breast milk but in very small quantities, insufficient to be considered harmful.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Emedon® Tablet: Each box contains 10 x 10’s tablets in blister pack.

-
Etorgin®
Presentation:
Etorgin® 90 Tablet: Each film coated tablet contains Etoricoxib INN 90 mg.
Etorgin® 120 Tablet: Each film coated tablet contains Etoricoxib INN 120 mg.
Pharmacological action: Etoricoxib is a Non-Steroidal Anti-Inflammatory Drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities. It is a potent, orally active, highly selective cyclooxygenase -2 (COX-2) inhibitor within and above the clinical dose range. COX-2 has been shown to be primarily responsible for the synthesis of prostanoid mediators of pain, inflammation and fever. Selective inhibition of COX-2 by Etoricoxib decreases these clinical signs and symptoms with decreased GI toxicity and without effects on platelet function.
Indications: Etoricoxib is indicated for relief of pain and inflammation in - osteoarthritis, rheumatoid arthritis, other chronic musculoskeletal disorders, acute gout, dysmenorrhea & following dental surgery.
Dosage and directions for use: Adult and adolescent over 16 years: In case of osteoarthritis, dysmenorrhoea, chronic musculoskeletal disorders, 60 mg once daily. In case of rheumatoid arthritis 90 mg once daily. In case of pain following dental surgery, acute gout 120 mg once daily. Safety and effectiveness of Etoricoxib in paediatric patients have not been established.
Side Effects: Dry mouth, taste disturbance, mouth ulcers, flatulence, constipation, appetite and weight changes, chest pain, fatigue, paraesthesia, influenza-like syndrome & myalgia.
Precautions: Decreased kidney function and liver function, dehydration, hypertension, history of heart failure, perforation and people over 65 years of age.
Contraindications: Patients with known hypersensitivity to Etoricoxib or to any of the excipients of this medicinal product, active peptic ulceration or gastro-intestinal bleeding, severe hepatic dysfunction, children under 16 years of age, inflammatory bowel disease, uncontrolled hypertension, breast feeding.
Drug Interaction: Warfarin, ACE Inhibitor, Rifampicin, Lithium, Birth control pills, Methotrexate, Digoxin.
Use in Pregnancy & Lactation: Should be avoided in late pregnancy because it may cause premature closure of the ductus arteriosus. It should be used during the first two trimesters of pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether this drug is excreted in human milk.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Etorgin® 90 Tablet: Each Box contains 3 x 10’s tablets in Alu-Alu blister pack.
Etorgin® 120 Tablet: Each Box contains 3 x 10’s tablets in Alu-Alu blister pack.

-
Florixe®
Presentation:
Florixe® Cream: Each gram of cream contains Permethrin INN 50 mg.
Pharmacological action: Permethrin is a photo stable synthetic pyrethroid that possesses a broad spectrum of insecticidal activity and is generally rated as the safest insecticide because of its low primary toxicity. Along with the pediculicidal activity, Permethrin has ovicidal and scabicidal activity. The insect rapidly absorbs Permethrin via mouth, respiratory tract or the intact cuticle. Although the primary target tissue is the nervous system, insects are killed by a complex series of reactions in various organs such as metabolic exhaustion and paralysis of the nervous system.
Indications: Permethrin cream is indicated for the treatment of scabies.
Dosage and directions for use: Adults and children (over 12 years) - A full tube
- Children aged 6-12 years: up to half of a tube.
- Children aged 1-5 years: up to one fourth of a tube.
- Children aged 2 months to 1 year: up to one eighth of a tube.
Patients of > 2 months of age can use this cream. Cream should be applied to clean, dry and cool skin. If the body is hot due to warm bath or any other reason, skin should be allowed to cool down. It should be applied to the whole body excluding head. The whole body should be washed thoroughly 8-12 hours after treatment. Adults and children above 12 years will use a full tube as a single dose. If necessary maximum two tubes can be used as a single dose. The cream should not be applied to the vicinity of mouth and areas close to the eyes.
Side Effects: In scabies patients, skin discomfort, usually described as burning, stinging or tingling occurs in a few individuals soon after the cream is applied. Other transient effects are erythema, numbness, rash and pruritus.
Precautions: Permethrin is not an eye irritant, but the cream itself may cause marked irritation. Nursing staff who routinely apply Permethrin, may wear gloves to avoid any possible irritation to the hands.
Contraindications: Permethrin is contraindicated in patients with known hypersensitivity to the product, its components, other pyrethroids or pyrethrins.
Drug Interaction: The treatment of eczematous like reactions with corticosteroids should be withheld prior to treatment with Permethrin, as there is a risk of exacerbating the scabies infestation by reducing the immune response to the mite.
Use in Pregnancy & Lactation: There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed. It is not known whether Permethrin is excreted in human milk. Because many drugs are excreted in human milk, consideration should be given to discontinuing nursing temporarily or withholding the drug while the mother is nursing.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Florixe® Cream: Each box contains one tube of 30g cream.

-
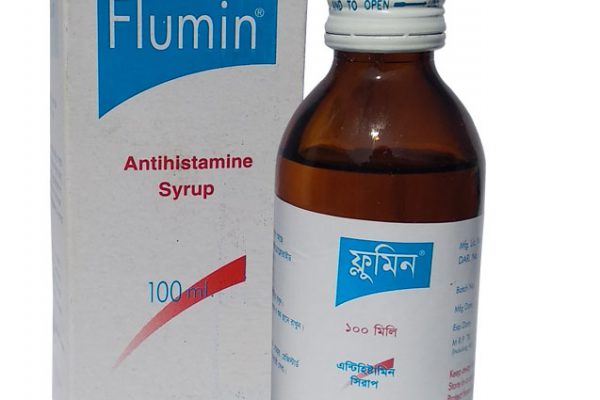
Flumin®
Presentation: Flumin® Syrup: Each 5 ml syrup contains Promethazine Hydrochloride USP 5 mg. Pharmacological action: Promethazine, a phenothiazine derivative, is a long acting antihistamine with mild atropine-like anticholinergic effects and some antiserotonin effects, and because of its marked effect on the central nervous system (CNS), it acts as an antiemetic, hypnotic, tranquilliser, and a potentiator of anaesthetics, hypnotics, sedatives and analgesics. Indications: Promethazine is an antihistamine used to treat different types of allergy symptoms, including itching, runny nose, sneezing, itchy or watery eyes, hives, and itchy skin rashes. Also it is used as a sedative and sleep aid for all types of patients and prevent or control nausea and vomiting, treat motion sickness. Dosage and directions for use: Allergic disorder: Children: 6 - 12 years: 10 to 25 ml as a single dose at night, or 10 ml two to three times daily. Children: 2-5 years: 5 to 15 ml as a single dose at night, or 5 ml two to three times daily, Sedation: Children: 6-12 years: 10 to 25 ml as a single dose at night. Children: 2-5 years: 5 to 15 ml as a single dose at Night, Travel sickness: Children: 6-12 years: 10 ml. Children: 2-5 years: 5 ml. To be taken the night before travel and repeated after 6-8 hours on the following day if required. Nausea and vomiting: Children: 6-12 years: 10 ml every 4 to 6 hours to a maximum daily dose of 25 ml. Children: 2 – 5 years: 5 ml every 4 to 6 hours to a maximum daily dose of 15 ml. Side Effects: Side effects may be seen in a few patients: drowsiness, dizziness, restlessness, headaches, nightmares, tiredness, and disorientation. Anticholinergic side effects such as blurred vision, dry mouth and urinary retention occur occasionally. Infants are susceptible to the anticholinergic effects of promethazine, while other children may display paradoxical hyperexcitability. The elderly are particularly susceptible to the anticholinergic effects and confusion due to promethazine. Precautions: Promethazine should be used with caution in patients with asthma, bronchitis or bronchiectasis, severe coronary artery disease, narrow angle glaucoma, epilepsy or hepatic and renal insufficiency. The use of promethazine should be avoided in children and adolescents with signs and symptoms suggestive of Reye's Syndrome. Contraindications: Promethazine should not be used in patients in coma or suffering from CNS depression of any cause. Promethazine should not be given to patients with a known hypersensitivity to promethazine or to any of the excipients. Promethazine is not allowed to children under 2 years of age. Drug Interaction: Promethazine will enhance the action of any anticholinergic agent, tricyclic antidepressant, sedative or hypnotic. Alcohol should be avoided during treatment. Promethazine may interfere with immunological urine pregnancy tests to produce false-positive or false-negative results. Promethazine should be discontinued at least 72 hours before the start of skin tests as it may inhibit the cutaneous histamine response thus producing false-negative results. Use in Pregnancy & Lactation: Should not be used during pregnancy, becoming pregnancy or lactating without telling your doctor. Do not use before breast-feeding without doctor's advice. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Flumin® Syrup: Each bottle contains 100 ml syrup with a measuring spoon.
-
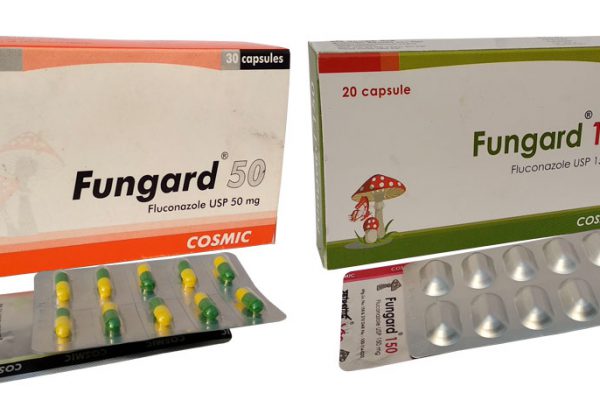
Fungard®
Presentation: Fungard® 50 Capsule: Each capsule contains Fluconazole USP 50 mg. Fungard® 150 Capsule: Each capsule contains Fluconazole USP 150 mg. Pharmacological action: Fluconazole is a triazole antifungal agent. It is a potent and selective inhibitor of fungal cytochrome P450 dependent enzymes necessary for the synthesis of ergosterol. The bioavailability of Fluconazole is unaffected by gastric pH. The plasma half life is approximately 30h.Most of the dose of Fluconazole is excreted unchanged in the urine, the reminder of the dose being eliminated in the urine as metabolites. Indications Fluconazole is indicated in the treatment of acute or recurrent vaginal candidiasis, mucosal candidiasis (as oropharyngeal candidiasis, oesophagitis, candiduria), systemic candidiasis and cryptococcal infections (including meningitis). Other indications-Fungal urinary tract infections, disseminated candidiasis, prophylaxis for fungal infection in neutropenic cancer patients, acute treatment of other systemic fungal infections such as coccidioidomycosis and histoplasmosis. Dosage and directions for use: Since oral absorption is rapid and almost complete, the daily dose of Fluconazole is the same for oral and intravenous administration. Oropharyngeal Candidiasis: The recommended dosage of fluconazole for oropharyngeal candidiasis is 200 mg on the first day, followed by 100 mg once daily for 7-14 days. Esophageal Candidiasis: The recommended dosage of fluconazole for esophageal candidiasis is 200 mg on the first day, followed by 100 mg once daily for 2-3 weeks. Systemic Candidiasis and Cryptococcal Meningitis: 400 mg daily followed by 200 mg once daily. The recommended duration of treatment for initial therapy of cryptococcal meningitis is 10 to 12 weeks. Acute or recurrent vaginal candidiasis: Single dose of 150 mg. Urinary Tract Infections and Peritonitis: 50 to 200 mg once daily. Prophylaxis in Patients Undergoing Bone Marrow Transplantation: 400 mg once daily. Side Effects: Nausea, abdominal discomfort, diarrhoea, flatulence, headache, rash, less frequently dyspepsia, vomiting, abnormalities in liver enzymes, seizures, alopecia and Stevens Johnson syndrome reported. Precautions: Cautions should be taken in renal impairment; in hepatic disease liver function should be monitored and should be discontinued if signs or symptoms of hepatic disease appear. Contraindications: Fluconazole should not be used in patients with known hypersensitivity to Fluconazole or to related triazole compounds. Drug Interaction: Rifampicin reduces plasma concentration of Fluconazole. Effects of nicoumalone, phenytoin and warfarin are enhanced. Plasma concentrations of sulphonylureas & theophyline are possibly increased. Use in Pregnancy & Lactation: There are limited data on the use of Fluconazole in pregnant woman. However Fluconazole should be used in pregnancy only when the benefit clearly outweighs the risk. Fluconazole is excreted in breast milk in levels about half of those found in plasma; therefore the drug should be avoided during lactation. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Fungard® 50 Capsule: Each box contains 3 x 10’s capsules in blister pack. Fungard® 150 Capsule: Each box contains 2 x 10’s capsules in Alu-Alu blister pack.
-
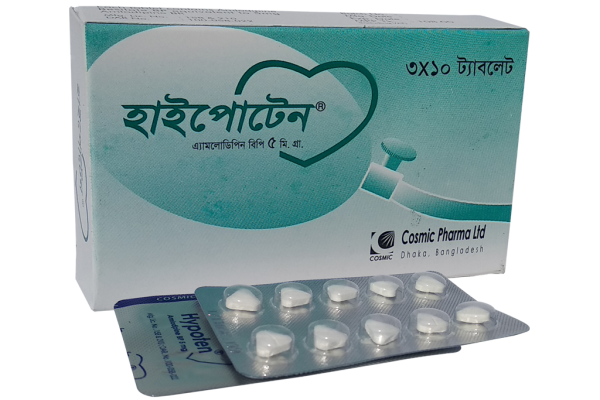
Hypoten®
Presentation:
Hypoten® Tablet: Each tablet contains Amlodipine 5 mg as Amlodipine Besilate BP.
Pharmacological action: Amlodipine is a calcium antagonist of the dihydropyridine group and inhibits the transmembrane influx of calcium ion into cardiac & smooth muscle. Amlodipine is slowly and incompletely absorbed, with 60-80% of an oral dose reaching the systemic circulation. Plasma half-life ranges from 30-60 hrs (mean 35.7 hrs). Amlodipine is extensively metabolized in the liver prior to excretion with only about 5% unchanged drug excreted in the urine.
Indications: Amlodipine is indicated in patients with mild to moderate hypertension (alone or in combination with other antihypertensives), treatment of chronic stable and vasospastic angina, Raynaud's disease.
Dosage and directions for use: For treatment of both hypertension and angina pectoris, the usual initial dose is 5 mg once daily. If the desired therapeutic effect cannot be achieved within 2-4 weeks, the dose may be increased to a maximum dose of 10 mg once daily. Amlodipine 10 mg once daily provides symptomatic improvement in patients with Raynaud's disease. Children with hypertension from 6 years to 17 years of age 2.5 mg once daily as a starting dose, up-titrated to 5 mg once daily if blood pressure goal is not achieved after 4 weeks. Doses in excess of 5 mg daily have not been studied in pediatric patients. Children under 6 years old the effect of amlodipine on blood pressure in patients less than 6 years of age is not known.
Side Effects: Amlodipine is generally well tolerated. The most commonly observed side effects are headache, peripheral oedema, palpitations, flushing, dizziness, nausea, abdominal pain.
Precautions: Caution should be taken in patients with hepatic impairment, congestive heart failure, sinus node disease, aortic stenosis, beta-blocker withdrawal, during pregnancy and breast-feeding.
Contraindications: Amlodipine is contra-indicated in patients with known hypersensitivity to dihydropyridine derivatives.
Drug Interaction: Digoxin: Absence of any interaction between amlodipine and digoxin in healthy volunteers has been documented in a controlled clinical study. Warfarin: An unpublished study in healthy volunteers indicates that amlodipine does not significantly alter the effect of warfarin on prothrombin time. Cimetidine: An unpublished clinical study indicated no interaction between amlodipine & cimetidine in healthy volunteers. Food: Food does not alter the rate or extent of absorption of amlodipine.
Use in Pregnancy & Lactation: Safety in pregnancy has not been established. It is not known whether Amlodipine is excreted in breast milk. It is advised to stop breastfeeding during treatment with Amlodipine.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Hypoten® Tablet: Each box contains 3 x 10’s tablets in blister pack.

-
Levocos®
Presentation: Levocos® 500 Tablet: Each film coated tablet contains Levofloxacin 500 mg as Levofloxacin Hemihydrate USP. Pharmacological action: Levofloxacin is a synthetic, broad-spectrum antibacterial agent. It kills bacteria by blocking bacterial DNA synthesis by inhibiting bacterial DNA gyrase (Topoisomerase-II). Indications: It is indicated for the treatment of adults with acute maxillary sinusitis, acute bacterial exacerbation of chronic bronchitis, community acquired pneumonia, skin & skin structure infections as abscess, cellulitis, furuncles, impetigo, pyoderma, wound infections, complicated & uncomplicated urinary tract infections, acute pyelonephritis. Dosage and directions for use: Acute sinusitis: 500 mg once daily for 10-14 days. Exacerbation of chronic bronchitis: 250-500 mg daily for 7 days. Community acquired pneumonia: 500 mg once or twice daily for 7-14 days. Complicated urinary-tract infections: 250 mg once daily for 7-10 days. Uncomplicated urinary tract infections: 250 mg once daily for 3 days. Acute pyelonephritis: 250 mg daily for-10 days. Uncomplicated skin & skin structure infections: 500 mg once daily for 7-10 days. Typhoid fever: 500 mg once daily for 7-14 days. Side Effects: Side effect includes- Nausea, diarrhea, vaginitis, pruritus, abdominal pain, dizziness, flatulence, rash, dyspepsia, insomnia, moniliasis, taste perversion, vomiting. Precautions: While taking Levofloxacin, adequate amount of water should be drunk to avoid risk of crystalluria. Dose adjustment should be exercised during Levofloxacin ingestion in presence of renal insufficiency. Contraindications: Levofloxacin is contraindicated in patients with a history of hypersensitivity to Levofloxacin, quinolones antimicrobial agents, or any other components of this product. Drug Interaction: Antacids, Iron and Adsorbents reduce absorption of Levofloxacin. NSAIDs may increase risk of CNS stimulation. Warfarin may increase the risk of bleeding. Use in Pregnancy & Lactation: Should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Levofloxacin is excreted into human milk so a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Levocos® 500 Tablet: Each box contains 3 x 10’s tablets in blister pack.
-
Licod-M®
Presentation: Licod-M® Syrup:Each 5 ml syrup contains Vitamin-A BP (as Vitamin A Propionate) 2000 IU, Vitamin-D3 (as Cholecalciferol BP) 200 IU, Vitamin-B1 (as Thiamine Hydrochloride BP) 0.70 mg, Vitamin -B2 (as Riboflavin-5-Sodium Phosphate BP) 0.85 mg, Vitamin-B6 (as Pyridoxine Hydrochloride BP) 0.35 mg, Vitamin-C (as Ascorbic Acid BP) 17.50 mg, Vitamin-E (as Alpha -Tocopherol BP) 1.50 mg, Nicotinamide BP 9.00 mg, and Cod Liver Oil BP 100.00 mg. Pharmacological action:Licod-Mcontains eight essential Vitamins with Cod Liver Oil. It provides extra protection for thechildren. Licod-Mensures for getting enough Vitamin that help children to be grown up strong and healthy.Cod Liver Oil contains vitamin-A, vitamin -D, EPA & DHA. Vitamin -A is essential for the immunesystem, bone growth, night vision, cellular growth, testicular and ovarian function. Vitamin-D is essentialfor the absorption and utilization of Calcium which is also required for skeletal growth. EPA and DHAbeing Omega-3 fatty acids are converted in the body to produce prostaglandins that affect on the action ofhormones. Omega-3 fatty acids which relieve the symptoms of osteoarthritis, rheumatoid arthritis enhanceimmune function and promote healthy blood circulation. It is thought that EPA and DHA may reduce therisk of coronary heart disease. DHA seems to be essential for normal brain development in unborn babies. Indications:Ithelps to prevent vitamin deficiency and to restore lost Vitamin after illness, lost appetite or just when feel tired, restless or run down in growing children. It also helps to maintain healthy skin, hair, nails, teeth and bones. Dosage and directions for use: Infants (1-12 Months): ½ Teaspoonful/day. Child (1-4 Years): 1 Teaspoonful/day. Child (4 Years up): 1½ Teaspoonful/day. Adult : 2 Teaspoonful/day. Note : Can be taken with water or milk. Side Effects:Generally well tolerated. However, a few allergic reactions may be seen. Precautions:This medicine may accumulate in the body, which cause danger. So, it should not use over dosage or use continuously except recommended by physicians. Contraindications: Should not be used in the patients in levodopa therapy, hypocalcaemia, glucose-6-phosphate dehydrogenase deficiency and hypersensitivity to any of the active ingredients. Drug Interaction: Erythromycin, Conjugated estrogens, Sodium Bicarbonate, Chloramphenicol, Chlorothiazide etc. Use in Pregnancy & Lactation:Should be taken on physician advice if clearly needed. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Licod-M® 100 ml: Each bottle contains 100 ml syrup with a measuring spoon. Licod-M® 200 ml: Each bottle contains 200 ml syrup with a measuring spoon.
-
Lixidol®
Presentation: Lixidol®Tablet:Each film coated tablet contains Ketorolac Tromethamine USP 10 mg. Pharmacological action:Ketorolac Tromethamine is a non-steroidal anti-inflammatory drug (NSAID) that exhibits analgesic, anti-inflammatory, anti-pyretic activity. Ketorolac Tromethamine inhibits synthesis of prostaglandins and may be considered a peripherally acting analgesic. The biological activity of Ketorolac Tromethamine is associated with the S-form. Ketorolac tromethamine possesses no sedative or anxiolytic properties.Pharmacokinetic property of Ketorolac Tromethamine is linear. It is highly protein bound and is largely metabolized in liver. The products of metabolism and some unchanged drugs are excreted in the urine. Indications:Ketorolac is indicated for the short term management of moderate to severe acute post-operative pain, dental pain, orthopaedic pain, renal colic, nephrolithiasis, cancer pain, musculo-skeletal pain. Dosage and directions for use:Adult (Under 65 years of age): The usual oral dose is 10 mg every 4 to 6 hours, as required. Doses exceeding 40 mg per day is not recommended. Elderly (65 years of age and older): The usual oral dose is 10 mg every 6 to 8 hours. Daily doses of 30 mg to 40 mg per day should not be exceeded. The use of Ketorolac tablets is recommended for the shortest possible time. Side Effects:It is generally well tolerated. However, side effects like dry mouth, excessive thirst, psychotic reactions, convulsion, myalgia, hyponatremia, hyperkalemia, raised blood urea and creatinine, renal failure, hypertension, bradycardia, chest pain, purpura, post operativehaemorrhage, haematoma, liver function change etc. may occur in some cases. Gastrointestinal side-effects include abdominal discomfort, constipation, diarrhea, dyspepsia, flatulence, fullness, gastritis, gastrointestinal bleeding, gastrointestinal pain, nausea, pancreatitis, peptic ulcer, perforation, stomatitis, vomiting etc. Precautions:Precaution should be taken in elderly, allergic disorder, renal, cardiac & hepatic impairment, porphyria, patient with low body weight (<50 kg). Contraindications:Known hypersensitivity to the drug, asthma, syndrome of rhinitis and nasal polyposis, active peptic ulceration or gastrointestinal bleeding, pre-renal conditions including renal artery stenosis, hypovolemia or dehydration, disorders of coagulation or platelet function, cirrhosis of liver or ascites,suspected or confirmed cerebrovascular bleeding, concomitant use with NSAIDs. Drug Interaction:Methotrexate, diuretics, ACE inhibitors, warfarin, lithium, aminoglycosides, antiepileptic drugs, psychoactive drugs and other NSAIDs. Use in Pregnancy & Lactation:The safety of Ketorolac in human pregnancy has not been established. It is therefore contraindicated during pregnancy, labor or delivery. As Ketorolac has been detected in human milk at low levels, it is also contraindicated in mothers who are breast-feeding. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Lixidol®Tablet: Each box contains 3 x 10’s tablets in Alu-Alu blister pack.
-
Melafresh Cream
P Presentation: Melafresh Cream: Fluocinolone acetonide 0.01%, Hydroquinone USP 4% and Tretinoin USP 0.05%. Pharmacology: Melafresh Cream is the combination of three active ingredients. Fluocinolone Acetonide is a corticosteroid for topical dermatological use and is classified therapeutically as an anti-inflammatory. Hydroquinone is classified therapeutically as a depigmenting agent and may interrupt one or more steps in the tyrosine-tyrosinase pathway of melanin synthesis. Tretinoin is a metabolite of Vitamin A which acts as a keratolytic agent. Indications: Melafresh cream is indicated for the short-term treatment of moderate to severe melasma of the face. After starting the treatment avoid sunlight exposure is recommended. Use a sunscreen or sunblock. Dosage and Directions for use: Melafresh cream should be applied once daily at night. It should be applied at least 30 minutes before bedtime. Rinse the face gently and dry it. A thin film of the cream should be applied to the hyperpigmented areas of melasma including about ½ inch of normal appearing skin surrounding each lesion. Melafresh cream is for short-term (up to 8 weeks) treatment should not for long-term (more than 8 weeks) or maintenance of moderate to severe melasma on the face. During the day use a sunscreen or sunblock and avoid sunlight exposure. Side-effects: Common side-effects include redness, burning, itching, irritation, dryness, allergic contact dermatitis and secondary infection at the site of application. The majority of these side-effects may be mild to moderate in severity. Precautions: This cream contains Hydroquinone and Tretinoin that may cause mild to moderate irritation such as skin reddening, peeling, mild burning sensation and dryness. Transient skin reddening or mild burning sensation does not preclude treatment. If a reaction suggests hypersensitivity or chemical irritation, the use of the medication should be discontinued. Manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced by systemic absorption of topical corticosteroid while on treatment. If HPA axis suppression is noted, the use of this cream should be discontinued. Contra-indications: Melafresh cream is contraindicated in individuals with a history of hypersensitivity, allergy, or intolerance to this product or any of its components. Drug Interactions: Patients should avoid medicated or abrasive soaps and cleansers, soaps and cosmetics with drying effects, products with high concentration of alcohol and astringent and other irritants or keratolytic drugs while on cream treatment. Pregnancy and lactation: Pregnancy: Pregnancy Category C. This cream contains the Tretinoin, which may cause embryo-fetal death, altered fetal growth, congenital malformations and potential neurologic deficits. There are no adequate and well-controlled studies in pregnant women. This cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Lactation: It is not known whether topical application of this cream could result in sufficient systemic absorption to produce detectable quantities of Fluocinolone Acetonide, Hydroquinone, or Tretinoin in human milk. Because many drugs are secreted in human milk, caution should be exercised when cream is administered to a nursing woman. Pharmaceutical precaution : Store in a cool and dry place, protected from light. Do not freeze. Keep out of the reach of children. Commercial Packaging: Melafresh Cream: Each Box contains one tube of 30gm cream.
-
Mucopront®
Presentation: Mucopront® Syrup: Each 5 ml syrup contains Bromhexine Hydrochloride BP 4mg. Pharmacological action: Bromhexine is an oral mucolytic agent with a low level of associated toxicity. It acts on the mucus at the formative stages in the glands, within the mucus-secreting cells. Bromhexine disrupts the structure of acid mucopolysaccharide fibres in mucoid sputum and produces less viscous mucus, which is easier to expectorate. Bromhexine hydrochloride is rapidly absorbed from the gastrointestinal tract. It is widely distributed to body tissues and is highly bound to plasma proteins. About 85 to 90% of a dose is excreted in the urine mainly as metabolites. It has a terminal elimination half-life of up to about 12 hours. Indications: Bromhexine is indicated in the treatment of respiratory disorders associated with productive cough. These include: tracheobronchitis, bronchitis with emphysema, bronchiectasis, bronchitis with bronchospasm, chronic inflammatory pulmonary conditions & pneumoconiosis. Dosage and directions for use: Adults: The recommended daily dose is 2 to 4 teaspoonful 3 times daily. Initially 4 teaspoonful 3 times daily and then as required. Children: Suggested dosage for children under 2 years is ¼ teaspoonful 3 times daily, for 2-5 years ½ teaspoonful 3 times daily and for children aged 5-10 years 1 teaspoonful 3 times daily. Side Effects: Gastrointestinal side effects may occur occasionally with Bromhexine and a transient rise in serum aminotransferase values has been reported. Other reported adverse effects include headache, dizziness, sweating and skin rash. Precautions: Since mucolytics may disrupt the gastric mucosal barrier, bromhexine should be used with caution in patients with a history of gastric ulceration. Clearance of bromhexine or its metabolites may be reduced in patients with severe hepatic or renal impairment. Contraindications: Bromhexine is contraindicated for use in patients with known hypersensitivity or idiosyncratic reaction to bromhexine hydrochloride (or any of the other ingredients in the product). Drug Interaction: Not known. Use in Pregnancy & Lactation: Pregnancy Category B: Bromhexine has been taken by a large number of pregnant women and women of child bearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed. It is not known whether Bromhexine is excreted in breast milk or whether it has a harmful effect on the breast-feeding infant. Therefore it is not recommended for breast-feeding mothers unless the potential benefits to the patient are weighed against the possible risk to the infant. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Mucopront® Syrup: Each bottle contains 100 ml syrup with a measuring spoon.
-
Neu-3®
Presentation: Neu-3® Tablet: Each film coated tablet contains Thiamine Mononitrate (Vit-B1) BP 100 mg, Pyridoxine Hydrochloride (Vit-B6) BP 200 mg, Cyanocobalamin (Vit-B12) BP 200 mcg. Pharmacological action: Neu-3® is a combination of Vitamin B1 (Thiamine), Vitamin B6 (Pyridoxine) & Vitamin B12 (Cyanocobalamin). These vitamins play essential role as co-enzyme in the metabolism of nervous system. Thus this combination normalizes the nerve cell metabolism. These combination supports the regeneration of nerve fibers and myelin sheath by activation of the metabolism and the natural repair mechanism. Indications: Neu-3® is indicated in chronic pains like sciatica, lumbago, trigeminal neuralgia, facial paralysis, optic neuritis as well as neuropathy & cardiac complications. Besides these, deficiency exists of these relevant vitamins. Dosage and directions for use: 1-2 tablets three times a day or as directed by the physician. Side Effects: Generally well tolerated. Hypersensitive reaction and sensitivity disturbance may occur rarely. Precautions: Cyanocobalamin should not be given before a diagnosis has been fully established because of the masking symptoms of subacute degeneration of the spinal cord. Contraindications: It should not be used in patients on levodopa therapy and hypersensitivity to any of the above vitamins. Drug Interaction: No significant drug interactions have been reported but neomycin, chloramphenicol, aminosalicylic acid & histamine H2 receptor antagonist may reduce Vitamin B12 absorption. Use in Pregnancy & Lactation: Safe for pregnant woman. It is not known whether thiamine (Vitamin B1) is excreted in breast milk or not, but may be taken cautiously during lactation. Vitamin B6 & B12 have no adverse effect during lactation. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Neu-3® Tablet: Each box contains 3x10’s tablets in blister pack.
-
Nova Plus®
Presentation: Nova Plus® Tablet: Each tablet contains Paracetamol BP 500 mg and Caffeine BP 65 mg. Pharmacological action: Paracetamol is a fast acting and safe analgesic with marked antipyretic property. Caffeine is used in this product to increase the solubility and transmembrane permeation of paracetamol and increases the pain and fever relieving effects of Paracetamol. Caffeine has also an intrinsic power to raise vessel tone in the brain, which provides another benefit to treat migraine. Indications: It is recommended for the treatment of most painful and febrile conditions, for example, headache, including migraine, backache, toothache, neuralgia, rheumatic pain and dysmenorrhoea, and relief of the symptoms of colds, influenza and sore throat. Dosage and directions for use: Adults, including the elderly and children 12 years and over: 2 tablets every 4-6 hours or as recommended by the physicians. Do not exceed 8 tablets in 24 hours. Side Effects: This combination may cause skin rashes, peeling, mouth ulcer, nausea, sudden weight loss, neutropenia & gastro-intestinal disturbances etc. High dose administration may cause hepatotoxicity. Precautions: Should be given with caution in patients with hepatic or renal failure, in patients taking other hepatotoxic medication & prolonged use of the drug without consulting a physician should be avoided. Excessive intake of tea or coffee should be avoided while taking this product. Contraindications: Hypersensitivity to Paracetamol, Caffeine or any other components of it. Drug Interaction: The anticoagulant effect of warfarin and other coumarins may be enhanced by prolonged regular use of paracetamol with increased risk of bleeding; occasional doses have no significant effect. Risk of hepatotoxicity of Paracetamol may be increased in alcoholics or in patients taking other anti-epileptic medications. Use in Pregnancy & Lactation: This drug should be used during pregnancy only if clearly needed. Paracetamol and caffeine are excreted in breast milk but not in a clinically significant amount. Available published data do not contraindicate breast feeding. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Nova Plus® Tablet: Each box contains 10x10’s tablets in blister pack.
-
Nova®
Presentation: Nova® Tablet: Each tablet contains Paracetamol BP 500 mg. Nova® Suspension: Each 5 ml suspension contains Paracetamol BP 120 mg. Pharmacological action: Paracetamol is a fast acting and safe analgesic with marked antipyretic property. Paracetamol produces analgesic action by elevation of the pain threshold and antipyresis through action on the hypothalamic heat regulating centre. Indications: It is recommended temporarily relieves minor aches and pains due to: the common cold, headache, backache, minor pain of arthritis, toothache, muscular aches, premenstrual and menstrual cramps. Also reduces fever temporarily. Dosage and directions for use: Tablet: Adults, including the elderly and children 12 years and over: 2 tablets every 4-6 hours or as recommended by the physicians. Do not exceed 8 tablets in 24 hours. Children: (6-12 years) 1/2 to 1 tablet 3 to 4 times daily. For long term treatment, it is wise not to exceed the dose beyond 2.6 g/day. Suspension: Children Under 3 months: 10 mg/kg body weight (reduce to 5 mg/kg if jaundiced) 3 to 4 times daily. 3 months to below1 year: 1/2 to 1 teaspoonful 3 to 4 times daily. 1-5 years: 1/2 teaspoonful 3 to 4 times daily. 6-12 years: 2-4 teaspoonful 3 to 4 times daily. Adults: 4-8 teaspoonful 3 to 4 times daily. Side Effects: Paracetamol may cause skin rashes, peeling, mouth ulcer, nausea, sudden weight loss, neutropenia & gastro-intestinal disturbances etc. High dose administration may cause hepatotoxicity. Precautions: Should be given with caution in patients with hepatic or renal failure, in patients taking other hepatotoxic medication & prolonged use of the drug without consulting a physician should be avoided. Contraindications: Paracetamol is contraindicated in patients with a known hypersensitivity to its components. Drug Interaction: The anticoagulant effect of warfarin and other coumarins may be enhanced by prolonged regular use of paracetamol with increased risk of bleeding; occasional doses have no significant effect. Risk of hepatotoxicity of Paracetamol may be increased in alcoholics or in patients taking other anti-epileptic medications. Use in Pregnancy & Lactation: This drug should be used during pregnancy only if clearly needed. Though Paracetamol appears in breast milk & crosses the placenta it causes no harmful effect to breast-fed infants or foetus. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Nova® Tablet: Each box contains 20x10’s tablets in blister pack. Nova® Suspension: Each bottle contains 60 ml suspension with a measuring spoon.
-
Odofex®
Presentation: Odofex® 120 Tablet: Each film coated tablet contains Fexofenadine Hydrochloride USP 120 mg. Pharmacological action: Fexofenadine is a second-generation, long lasting H1-receptor antagonist which has a selective and peripheral H1-antagonistic action. Fexofenadine blocks the H1-receptor and thus prevents activation of cells by histamine in the GI tract, large blood vessels and bronchial smooth muscle. This leads to relief of the allergic symptoms. Unlike most other antihistamines, Fexofenadine does not enter into the brain from the blood and therefore, does not cause drowsiness. Indications: Fexofenadine is indicated for the relief of symptoms associated with seasonal and perennial allergic rhinitis and chronic idiopathic urticaria. Dosage and directions for use: Adults and Children 12 years and older-120 mg once daily or 180 mg once daily with water. Children 6 to 11 years- 30 mg twice daily or 60 mg once daily. Side Effects: Fexofenadine is generally well tolerated. The most commonly reported side effects are headache, dyspepsia, fatigue, drowsiness, nausea, chest tightness, dyspnoea etc. Precautions: Should be given with caution in patients with kidney disease or phenylketonuria because the effects may be increased because of slower removal of the medicine from the body. Contraindications: Fexofenadine is contraindicated in patients with known hypersensitivity to any of the ingredients. Drug Interaction: Co-administration of Fexofenadine Hydrochloride with either ketoconazole or erythromycin may cause increased plasma concentration of Fexofenadine. Antacid containing aluminium and magnesium may reduce the absorption of Fexofenadine. Fruit juices such as grapefruit, orange and apple may reduce the bioavailability of Fexofenadine. Use in Pregnancy & Lactation: There are no adequate and well controlled studies in pregnant women. Fexofenadine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Fexofenadine is excreted in human milk or not. Caution should be exercised when Fexofenadine is administered to a nursing woman. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Odofex® 120 Tablet: Each box contains 5x10’s tablets in blister pack.
-
Optimin®
Presentation: Optimin® Tablet: Each film coated tablet contains Meclizine HCl BP 25 mg and Pyridoxine HCl BP 50 mg. Pharmacological action: Meclizine is a histamine antagonist at H1 receptors, of the piperazine side chain group; it acts also on CNS by blocking muscarinic receptors (anticholinergic) in the brain. Pyridoxine is used to prevent uncontrolled excitation of CNS, and eventually uncontrolled muscle seizures. This combination provides a synergistic antiemetic effect. Indications: This combination is indicated for the management of nausea, vomiting, and dizziness during pregnancy or dizziness associated with motion sickness, vertigo associated with diseases affecting the vestibular system. Dosage and directions for use: The fixed-dose combination (FDC) is recommended for- Nausea & Vomiting: One tablet 1-2 times daily or as directed by physician. Two tablets at night followed by one tablet in the morning during pregnancy if necessary. Motion Sickness: The initial dose of one or two tablets should be taken one hour prior to embarkation for protection against motion sickness. Thereafter, the dose may be repeated every 24 hours for the rest of the journey. Vertigo: For the control of vertigo associated with diseases affecting the vestibular system, the recommended dose is one to four tablets in divided doses, 2-3 times daily depending upon clinical response. Side Effects: Drowsiness, dry mouth, and blurred vision on rare occasions. Precautions: Meclizine/Pyridoxine combination may cause drowsiness, so it is better not to operate dangerous machines during therapy. Due to its potential anticholinergic action, patient with asthma, bronchitis, emphysema, enlarged prostate, glaucoma or urinary tract blockade should take meclizine HCl (like other antiemetics) with caution. Contraindications: Hypersensitivity to Meclizine Pyridoxine or any other components of it. Drug Interaction: Meclizine may increase the toxicity with CNS depressants, neuroleptics and anticholinergic. Meclizine & Pyridoxine combination has a synergistic drowsiness effect with alcohol, sedatives, and tranquilizers. Use in Pregnancy & Lactation: Meclizine is a FDA pregnancy category B. Meclizine is not expected to be harmful to an unborn baby. Pyridoxine is a FDA pregnancy category B. Pyridoxine should be safe for use in pregnancy. Meclizine Hydrochloride may be distributed into breast milk. However problem in human have not been documented. On the other hand, Pyridoxine Hydrochloride has no adverse effects during lactation. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Optimin® Tablet: Each box contains 5x10’s tablets in blister pack.
-
Peptidin®
Presentation: Peptidin® 150 Tablet: Each film coated tablet contains Ranitidine 150mg as Ranitidine Hydrochloride USP. Pharmacological action: Ranitidine is a histamine H2-receptor antagonist. Ranitidine inhibit acid secretion by blocking H2-receptors on the parietal cell. As a result of its H2-receptor blocking action Ranitidine promotes rapid and effective ulcer healing with sustained pain relief, both day and night by reduction of gastric acid output. It reduces the amount of secreted gastric juice and hydrogen ion concentration and also reduces the amount of pepsin. Indications: Ranitidine is indicated in the treatment of Active duodenal ulcer, Benign gastric ulcer, Zollinger-Ellison syndrome, Gastroesophageal reflux disease (GERD), Erosive Esophagitis, & reduce gastric hyperacidity. Dosage and directions for use: The usual dose of Ranitidine in adults is- Active Duodenal Ulcer-150 mg twice daily, Maintenance of Healing of Duodenal Ulcers-150 mg once daily at bedtime, Zollinger-Ellison syndrome-150 mg twice daily, Benign Gastric Ulcer-150 mg twice daily, Maintenance of Healing of Gastric Ulcers-150 mg once daily at bedtime, GERD-150 mg twice daily, Erosive Esophagitis-150 mg four times daily, Hyperacidity-150 mg twice daily. Children: The recommended oral dose for the treatment of peptic ulcer in children is 2 mg/kg to 4mg/kg twice daily to a maximum of 300mg ranitidine per day. Side Effects: Side effects of Ranitidine are generally infrequent and minor. Headache, malaise, dizziness, constipation, nausea, abdominal pain and rash may occur. Mental confusion and hallucination have occurred in geriatric patients during ranitidine therapy. Precautions: Symptomatic response to Ranitidine therapy does not preclude the presence of gastric malignancy. Ranitidine should be given in reduced dosage to patients with impaired renal and hepatic function. Contraindications: Ranitidine are contraindicated in patients who are hypersensitive to this medication or to any of its ingredients. Drug Interaction: Increased or decreased prothrombin times have been reported during concurrent use of Ranitidine and Warfarin. Absorption of Ketoconazole is decreased when used with Ranitidine. Use in Pregnancy & Lactation: Safety of Ranitidine during pregnancy has not been established. This drug should be used during pregnancy only if clearly needed. Ranitidine is secreted in human milk, caution should be exercised when it is administered to nursing mother. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Peptidin® 150 Tablet: Each box contains 10x10’s tablets in Alu-Alu blister pack.
-
Pevicort®
Presentation: Pevicort® Cream: Each gram of cream contains Econazole Nitrate BP 10 mg and Triamcinolone Acetonide BP 1 mg. Pharmacological action: Econazole Nitrate is a broad spectrum antifungal agent. It is effective against various dermatophytes, yeasts and moulds. Moreover, it has activity against gram-positive bacteria. Triamcinolone Acetonide is a potent corticosteroid with anti-inflammatory, antipruritic and antiallergic activity. Indications: Pevicort® Cream is indicated in dermatomycosis caused by dermatophytol yeast and fungus with clear inflammatory and allergic symptoms such as various eczematos mycosis, diaper dermatitis, eczema marginatum, intertrigo, follicolitis, tricophytica and cycosis barbi. It is also indicated for the treatment of mycosis present in various folds of the body. Dosage and directions for use: The cream should be applied by gently rubbing into the skin with the finger once or twice daily for 14 days or as directed by a registered physician. Use with caution in infants and young children. The infected area should be kept clean and dry during treatment. Side Effects: Generally well tolerated, even to sensitive skin. Burning sensation, pruritis and redness of skin have rarely been reported. Precautions: Long term continuous steroid therapy should be avoided since adrenal suppression can occur, particularly when infants or children are treated or when occlusive dressings are applied. Discontinuation of the medication is advised if hypersensitivity occurs. Contraindications: Like all preparations containing corticosteroids, this cream should not be used on tubercular skin infections or in viral diseases (e.g. herpes, vaccinia, and varicella). Hypersensitivity has rarely been recorded. If it occurs, application of the product should be discontinued. Drug Interaction: Despite limited systemic availability after cutaneous application, clinically relevant interactions may occur and have been reported in patients taking oral anticoagulants, such as warfarin and acenocoumarol. In these patients, caution should be exercised and the INR should be monitored more frequently. Adjustment of the oral anticoagulant dosage may be necessary during the treatment with and after its termination. Use in Pregnancy & Lactation: The use of corticosteroids during pregnancy has not been established. Topical steroids should not be used extensively in pregnancy, i.e. in large amounts or for prolonged periods. Should not use in first trimester of pregnancy. Excretion in breast-milk is likely to be negligible. So it can be used topically/vaginally during lactation if needed. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Pevicort® Cream: Each box contains one tube of 10g cream.
-
Rabium®
Presentation: Rabium® Tablet: Each enteric coated tablet contains Rabeprazole Sodium INN 20 mg. Pharmacological action: Rabeprazole suppresses gastric acid secretion by inhibiting the gastric H+/K+ ATPase at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, By acting specifically on the proton pump, which blocks the final step in acid production, thus reducing gastric acidity. Rabeprazole has been characterized as a gastric proton-pump inhibitor. Indications: Rabeprazole is indicated for Healing of Erosive or Ulcerative Gastroesophageal Reflux Disease (GERD), Maintenance of Healing of Erosive or Ulcerative GERD, Treatment of Symptomatic GERD, Healing of Duodenal Ulcers, Helicobacter pylori eradication to reduce the risk of Duodenal Ulcer recurrence, Treatment of Pathological Hypersecretory conditions including Zollinger-Ellison Syndrome. In pediatric patients 1 to 11 years of age for treatment of GERD. Dosage and directions for use: Adults: The usual recommended dose is 20 or 40 mg tablet once daily. The majority of patients are healed within 4 to 8 weeks. For patients who do not heal after 4-8 weeks, an additional 4-8 weeks treatment may be considered. Children (aged 1-11 years) Dose: <15kg body weight- 5mg /day up to 12 weeks with the option to increase to 10 mg if inadequate response and >15kg body weight -10mg/day for up to 12 weeks. Elderly: Dose adjustment is not required in the elderly. Side Effects: Rabeprazole is generally well tolerated. The observed undesirable effects have been generally mild/moderate and transient in nature. The most common adverse events are headache, diarrhoea and nausea. The less common adverse effects are abdominal pain, asthenia, flatulence, rash, dry mouth etc. Precautions: Administration of Rabeprazole to patients with mild to moderate liver impairment results in increased exposure and decreased elimination. Caution should be exercised in patients with severe hepatic impairment. Contraindications: Rabeprazole Sodium is contraindicated in patient with known hypersensitivity to Rabeprazole or to any component in the product. Drug Interaction: Rabeprazole increased INR and prothrombin times which have been reported with concomitant use with warfarin , patients need to be monitored. Rabeprazole has been shown to inhibit cyclosporine metabolism in vitro. Rabeprazole inhibits gastric acid secretion and may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (e.g., ketoconazole, iron salts, digoxin, and mycophenolate mofetil) .Rabeprazole may reduce the plasma levels of atazanavir, & may increase serum level of methotrexate. Use in Pregnancy & Lactation: US FDA Pregnancy Category C. There are no adequate and well-controlled studies with Rabeprazole in pregnant women. Because of these findings, it should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known if Rabeprazole is excreted in human milk. Many drugs are excreted in milk, caution should be exercised when it is administered to a nursing woman. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Rabium® Tablet: Each box contains 5x10’s tablets in Alu-Alu blister pack.
-
Rhinex®
Presentation:Rhinex® Tablet: Each film coated tablet contains Desloratadine INN 5mg.
Pharmacological action: Desloratadine is a non-sedating, long-acting histamine antagonist with selective peripheral H1-receptor antagonist activity. After oral administration, desloratadine selectively blocks peripheral histamine H1-receptors because the substance is excluded from entry to the central nervous system.
Indications: Desloratadine is indicated in adults and adolescents aged 12 years or older for the relief of symptoms associated with allergic rhinitis & urticaria.
Dosage and directions for use: The recommended dose is one tablet once a day or as directed by the registered physician. The safety and efficacy of desloratadine film-coated tablets in children below the age of 12 years have not been established.
Side Effects: Desloratadine is generally well tolerated. Less serious side effects may include: dry mouth, sore throat, cough, muscle pain, drowsiness, tired feeling, nausea, diarrhea or headache.
Precautions: In the case of severe renal insufficiency, desloratadine should be used with caution. Desloratadine does not usually cause drowsiness when used at recommended doses. However, do not drive, use machinery, or do any activity that requires alertness until you are sure you can perform such activities safely.
Contraindications: Desloratadine are contraindicated in patients who are hypersensitive to this medication or to any of its ingredients or to loratadine.
Drug Interaction: No clinically relevant interactions were observed in clinical trials with desloratadine tablets in which erythromycin, ketoconazole, azithromycin, antihistamines, fluoxetine & cimetidine were co-administered.
Use in Pregnancy & Lactation: FDA pregnancy category C. Desloratadine should be used in pregnancy only if clearly needed. Desloratadine can pass into breast milk and may harm a nursing baby so the drug should be used with caution in nursing women.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Rhinex® Tablet: Each box contains 10x10’s tablets in blister pack.

-
Roxenole®
Presentation: Roxenole ® Tablet: Each tablet contains delayed release Naproxen 500 mg as Naproxen Sodium USP and immediate release Esomeprazole 20 mg as Esomeprazole Magnesium Trihydrate USP. Pharmacological action: Roxenole® consists of an immediate-release esomeprazole magnesium layer and an enteric-coated naproxen core. As a result, esomeprazole is released first in the stomach, prior to the dissolution of naproxen in the small intestine. Naproxen is a NSAID with analgesic and antipyretic properties. The mechanism of action of Naproxen is to inhibit the prostaglandin synthesis. Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. By acting specifically on the proton pump, it blocks the final step in acid production, thus reducing gastric acidity. Indications: Relief of signs and symptoms of osteoarthritis, rheumatoid arthritis , & ankylosing spondylitis and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID associated gastric ulcers. Dosage and directions for use: The recommended dose is one tablet twice daily. Use the lowest effective dose. It should be avoided in moderate/severe renal insufficiency or in severe hepatic insufficiency. Consider dose reduction in mild/moderate hepatic insufficiency. This drug is to be taken at least 30 minutes before meals. Side Effects: Generally well tolerated. Most common side effect are erosive gastritis, dyspepsia, gastritis, diarrhea, gastric ulcer, upper abdominal pain, nausea. Precautions: Treatment should be withdrawn when active and clinically significant bleeding from any source occurs. Serious gastrointestinal (GI) adverse events, which can be fatal. The risk is greater in patients with a prior history of ulcer disease or GI bleeding, and in patients at high risk for GI events, especially the elderly. So it should be used with caution in these patients & also in patients with fluid retention or heart failure. Serious and potentially fatal cardiovascular (CV) thrombotic events, myocardial infarction, and stroke. Patients with known CV disease/risk factors may be at greater risk. Contraindications: This combination is contraindicated in patients with known hypersensitivity to Naproxen, Esomeprazole, substituted benzimidazoles, or to any of the excipients. Also contraindicated in patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients. Drug Interaction: Concomitant use with warfarin may result in increased risk of bleeding complications. Concomitant use of NSAIDs may reduce the antihypertensive effect of ACE inhibitors, angiotensin II receptor antagonists, diuretics, and beta blockers. Esomeprazole inhibits gastric acid secretion and may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (e.g. ketoconazole, iron salts, erlotinib, digoxin, and mycophenolate mofetil). Patients treated with digoxin may need to be monitored for increases in digoxin toxicity. Avoid concomitant use of esomeprazole with clopidogrel. Use in Pregnancy & Lactation: US FDA Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. Because of these findings, it should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. In late pregnancy should be avoided. Should not be used in nursing mothers due to the Naproxen component. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Roxenole ® Tablet: Each box contains 3x10’s tablets in Alu-Alu blister pack.
-
Salol®
Presentation:Salol® Syrup: Each 5 ml syrup contains Salbutamol 2 mg as Salbutamol Sulphate BP.
Pharmacological action: Salbutamol is a selective β2-agonist bronchodilator which provides short acting bronchodilation in reversible airways obstruction. As a β-adrenergic stimulant for relief of bronchospasm such as occurs with asthma, bronchitis, emphysema. It has a highly selective action on the receptors in bronchial muscle and in therapeutic dosage, little or no action on the cardiac receptors. Salbutamol works by relaxing the muscles in the walls of the small airways in the lungs.
Indications: Salbutamol is used to rapidly treat asthma, bronchospasm and reversible airways obstruction by widening the airways of the lungs.
Dosage and directions for use: The recommended usual oral dose in- Adults and Children over 12 years: 1-2 teaspoon (2mg-4mg) syrup three or four times daily. Children of 6-12 years: 1 teaspoon (2mg) syrup three or four times daily. Children of 2-6 years: 1/2 teaspoon (1mg) syrup three or four times daily or as directed by the physician.
Side Effects: Common side effects include tremor of the hands, tension, restlessness and a rapid heartbeat. There have been occasional reports of headache, and very rare reports of muscle cramps.
Precautions: Caution should be taken before taking Salbutamol if you are diabetic, as this product may cause an increase in blood sugar levels. This effect may be further increased if you are also taking corticosteroids. Also if you have an irregular heartbeat / heart rhythm, including a very fast pulse, overactive thyroid, acute severe asthma, unstable asthma, have an enlarged prostate, as Salbutamol may cause difficulty in passing water.
Contraindications: Salbutamol are contraindicated in patients who are hypersensitive to this medication or to any of its ingredients. Salbutamol presentations should not be used for threatened abortion during the first or second trimester of pregnancy. Salbutamol is not contraindicated in patients under treatment with monoamine oxidase inhibitors (MAOIs).
Drug Interaction: The effects of this product may be altered by guanethidine, reserpine, methyldopa, tricyclic antidepressants. Salbutamol oral preparations and non-selective beta-blocking drugs, such as propranolol should not usually be prescribed together. Caution should be exercised during use with anaesthetic agents such as chloroform, cyclopropane, halothane and other halogenated agents.
Use in Pregnancy & Lactation: FDA pregnancy category C. Salbutamol should be used in pregnancy only if clearly needed. Salbutamol can pass into breast milk and may harm a nursing baby so the drug should be used with caution in nursing women.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Salol® Syrup: Each bottle contains 100 ml syrup with a measuring spoon.

-
Sectil-20 Cap
Sectil-20 Cap - Omeprazole - 20mg - 5x10's -
Sectil-40 Cap
Sectil-40 Cap - Omeprazole -40mg - 10x4's -
Sectil®
Presentation:Sectil® 20 Capsule: Each capsule contains Omeprazole BP 20 mg as enteric coated pellets.
Sectil® 40 Capsule: Each capsule contains Omeprazole USP 40 mg as enteric coated pellets.
Pharmacological action: Omeprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, which suppress gastric acid secretion by specific inhibition of the H+/K+-ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, Omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production.
Indications: Omeprazole is a proton pump inhibitor indicated for treatment in adults of duodenal ulcer and gastric ulcer, treatment in adults and children of gastroesophageal reflux disease (GERD) and maintenance of healing of erosive esophagitis. The safety and effectiveness of Omeprazole in pediatric patients <1 year of age have not been established.
Dosage and directions for use: The usual adult dose of Omeprazole is- Treatment of Active Duodenal Ulcer-20 mg once daily for 4 weeks. May require an additional 4 weeks treatment. Gastric Ulcer-40 mg once daily for 4 to 8 weeks. GERD-20 mg once daily for 4 to 8 weeks. Maintenance of Healing of Erosive Esophagitis-20 mg once daily. Pathological Hypersecretory -60 mg once daily (varies with individual patient conditions). GERD and Maintenance of Healing of Erosive Esophagitis (1 to 16 years of age) - Dose: 5 < 10 kg body weight 5 mg/day, 10<20 kg body weight 10mg/day and > 20 kg body weight 20mg/day.
Side Effects: Omeprazole is generally well tolerated. Most common adverse reactions in adults are headache, abdominal pain, nausea, diarrhea, vomiting, and flatulence.
Precautions: Symptomatic response does not preclude the presence of gastric malignancy. Atrophic gastritis has been noted with long-term therapy.
Contraindications: Known hypersensitivity to any component of the formulation or substituted benzimidazoles (angioedema and anaphylaxis have occurred).
Drug Interaction: Omeprazole can prolong the elimination of diazepam, warfarin, phenytoin, cyclosporine, disulfiram, benzodiazepines; so monitor and determine need for dose adjustments. Omeprazole may interfere with drugs for which gastric pH affects bioavailability (e.g. ketoconazole, iron salts, ampicillin esters, and digoxin). There is no evidence of an interaction with theophylline, propranolol, metoprolol, lidocaine, quinidine, amoxycillin or antacids.
Use in Pregnancy & Lactation: US FDA Pregnancy Category C. There are no adequate and well-controlled studies with Omeprazole in pregnant women. Because of these findings, it should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known if it is excreted in human milk. Many drugs are excreted in milk, caution should be exercised when it is administered to a nursing woman.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Sectil® 20 Capsule: Each box contains 10x10’s capsules in Alu-Alu blister pack.
Sectil® 40 Capsule: Each box contains 10x4’s capsules in Alu-Alu blister pack.

-
Suplical-D®
Presentation: Suplical-D® Tablet: Each film coated tablet contains Elemental Calcium 500mg as Calcium Carbonate BP and Vitamin D3 BP 200 IU. Pharmacological action: Suplical-D® provides Calcium and Vitamin D3 where Calcium is an essential element and plays vital roles in the body. It makes body’s framework stronger by building bone. Vitamin-D3 is also essential for healthy bones as it aids in calcium absorption from the GI tract. In addition to this it stimulates bone formation. Controlled clinical studies show that calcium and vitamin-D3 have synergistic effects on bone growth as well as in osteoporosis and fracture prevention. Indications: Calcium and Vitamin-D3 is used for the treatment of osteoporosis, osteomalacia, rickets, tetany, and parathyroid disease. Also used in raised calcium requirement for children and adolescents at times of rapid growth, inadequate intake of calcium in the diet due to malnutrition, prevention and treatment of osteoporosis, disorders of osteogenesis and tooth formation (in addition to specific treatment), latent tetany and during pregnancy and lactation. It is also used as routine supplement and phosphate binder in chronic renal failure. Dosage and directions for use: Adults, elderly and children above 12 years of age: 2 tablets per day, preferably one tablet in the morning and one tablet in the evening with plenty of water or as directed by the physician. Taking in full stomach ensures better absorption. Not recommended for children under 12 years. Side-effects: Flatulence, diarrhoea, constipation, upper GI discomfort etc. are rare manifestation. Hyper- calcaemia due to prolong use has rarely been reported. Precautions: Caution should be taken in patients with Renal impairment, Sarcoidosis, Hypercalcemia and Hypercalciuria, Cardiac disease. When hypercalcemia occurs, discontinuation of the drug is usually sufficient to return serum calcium concentrations to normal. Patients with a history of stone formation should also be recommended to increase their fluid intake. Contra-indications: Hypersensitivity to any of the components, hypocalcaemia resulting from overdose of Vitamin D, hyperparathyroidism, bone metastases, severe renal insufficiency, severe hypercalciuria, renal calculi etc. Drug Interactions: Oral calcium can reduce the absorption of tetracycline & fluoride preparations and minimum 3 hours time should be allowed between ingestion of these medications. Thiazide diuretics reduces the renal excretion of calcium. Phenytoin, barbiturates, glucocorticoids may induce metabolism of Vitamin D. Concomitant ingestion of certain foods like spinach, cereals, milk and its derivatives may reduce the intestinal uptake of calcium. Pregnancy and lactation: Can be given to pregnant and lactating mothers as per recommendation of physician. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Suplical-D® Tablet: Each box contains 6 x 10’s tablets in blister pack, 15’s tablets & 30’s tablets in a HDPE container.
-
Suplical®
Presentation: Suplical® 500 Tablet: Each film coated tablet contains Elemental Calcium 500mg as Calcium Carbonate BP. Pharmacological action: Calcium is an essential element and plays vital roles in the body. It makes body’s framework stronger by building bone. Calcium Carbonate that is used as dietary calcium supplement, and to sequester phosphorus in the intestine to reduce total body phosphate accumulation in chronic renal failure. Indications: Calcium Carbonate is indicated in raised Calcium requirement, e.g. during pregnancy and lactation and in children & adolescents at the time of rapid growth. Inadequate intake of Calcium in the diet due to malnutrition. Prevention and treatment of osteoporosis. Disorders of osteogenesis and tooth formation (in addition to specific treatment), Latent tetany. Dosage and directions for use: The recommended dose of Calcium Carbonate is in – Adult: One tablet (500 mg elemental Calcium) daily or as directed by the physician. Higher doses should not be taken unless recommended by the physician. Children: 250 mg elemental Calcium daily or as directed by the physician. Adolescents: 250-500 mg elemental Calcium daily or as directed by the physician. Side Effects: Mild gastrointestinal disturbances (e.g. flatulence, abdominal pain, constipation) may occur. Hypercalcaemia and alkalosis are rarely produced with large doses. Precautions: In mild hypercalciuria or renal failure or stone formation in urinary tract, adequate checks must be kept on urinary calcium excretion. If necessary the dosage should be reduced or calcium therapy discontinued. Contraindications: Hypersensitivity to the Calcium Carbonate , Hypercalcaemia and hyperparathyroidism, Renal calculi and nephrolithiasis, Zollinger-Ellision syndrome and other causes of gastric acid hypersecretion. Drug Interaction: Concurrent administration of Calcium Carbonate reduces total absorption and peak serum levels of ciprofloxacin. Calcium Carbonate may enhance the cardiac effects of digoxin and other cardiac glycosides if systemic hypercalcaemia occurs. It may interfere with the absorption of concomitantly administered tetracycline preparations. Modification of vitamin D therapy may be required to avoid hypercalcaemia when Calcium Carbonate is used as a phosphate binder in chronic renal failure. Use in Pregnancy & Lactation: Calcium containing drugs are used widely in pregnancy by way of oral calcium supplementation. Calcium Carbonate can be used in lactating women too. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Suplical® 500 Tablet: Each box contains 3x10’s tablets in blister pack.
-
Suprax®
Presentation: Suprax® 200 Capsule: Each capsule contains Cefixime 200 mg as Cefixime Trihydrate USP. Suprax® DS Capsule: Each capsule contains Cefixime 400 mg as Cefixime Trihydrate USP. Suprax® PFS: After reconstitution, each 5 ml suspension contains Cefixime 100 mg as Cefixime Trihydrate USP. Pharmacological action: Cefixime is a semi-synthetic, broad spectrum cephalosporin antibiotic of third generation for oral administration. It is a bactericidal antibiotic, kills bacteria by interfering in the synthesis of the bacterial cell wall. Cefixime is highly stable in the presence of beta-lactamase enzymes. Cefixime has marked in-vitro bactericidal activity against a wide variety of gram-positive and gram-negative organisms. Indications: Cefixime is indicated in Respiratory Tract Infections, Otitis Media, Typhoid, Urinary Tract Infections, Uncomplicated gonorrhea, Parenteral-to-oral switch therapy. Dosage and directions for use: The usual course of treatment is 7 days. This may be continued for up to 14 days if required. The daily dose can be given in 1–2 divided doses. Adult and child over 10 years: 200–400 mg daily, child over 6 months: 8 mg/kg daily or 6 months–1 year: 75 mg (3.75 ml) daily, 1–4 years: 100 mg (5 ml) daily, 5–10 years: 200 mg (10 ml) daily. Typhoid and Paratyphoid: 200 mg 12 hourly for adult, 10 mg/kg 12 hourly for children. Gonorrhoea: 400-800 mg as a single dose for adult. The safety and efficacy of Cefixime has not been established in children less than 6 months. Directions for Reconstitution Suspension: To prepare suspension, add sufficient quantity boiled & cooled water, mentioned in the individual product’s label. Add the total water required in two portions. Shake the bottle well in each addition until all the powder is in suspension. Shake the bottle well before use suspension. A cup is supplied to aid water measuring and corrects dosing of suspension. Use within 7 days after reconstitution. Side Effects: Cefixime is generally well tolerated. The majority of adverse reactions observed in clinical trials were mild and self-limiting in nature. Among them diarrhoea, changes in the color of stool, nausea, abdominal pain, dyspepsia, flatulence, headache & dizziness have been reported. Precautions: Cefixime should be administered with caution in patients with markedly impaired renal function or a history of gastrointestinal diseases, particularly colitis. Cefixime should be given with caution to penicillin-sensitive patients as there is some evidence of partial cross-allergenicity between the penicillins and cephalosporins. Contraindications: Patients with known hypersensitivity to Cefixime or Cephalosporin group of drugs. After intake the drug if severe diarrhoea occurs, Cefixime should be discontinued. Drug Interaction: When Cefixime is administered concomitantly increased prothrombin time with or without clinical bleeding has been reported. Elevated carbamazepine levels have been reported in postmarketing experience when Cefixime is administered concomitantly. Use in Pregnancy & Lactation: Pregnancy Category B. There are no adequate and well-controlled studies in pregnant women. So this drug should be used during pregnancy only if clearly needed. It is not known whether Cefixime is excreted in human milk. So it is probably best either to avoid using the drug by nursing mother or to discontinue breast feeding. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Suprax® 200 Capsule: Each box contains 3x4’s capsules in Alu-Alu blister pack. Suprax® DS Capsule: Each box contains 2x4’s capsules in Alu-Alu blister pack. Suprax® PFS: Each bottle contains dry powder for 50ml oral suspension with a measuring cup & dropper.
-
Surgeflox®
Presentation: Surgeflox® 250 Capsule: Each capsule contains Flucloxacillin 250 mg as Flucloxacillin Sodium BP. Surgeflox® 500 Capsule: Each capsule contains Flucloxacillin 500 mg as Flucloxacillin Sodium BP. Surgeflox® PFS: After reconstitution, each 5 ml suspension contains Flucloxacillin 125 mg as Flucloxacillin Sodium BP. Pharmacological action: Flucloxacillin is an isoxazolyl penicillin which combines the properties of resistance to hydrolysis by penicillinase, gastric acid stability and activity against gram-positive bacteria. Flucloxacillin is a bactericidal antibiotic that is particularly useful against penicillinase producing staphylococci. Flucloxacillin kills bacteria by inhibiting the formation of cell wall of bacteria. Indications: Flucloxacillin is indicated for the treatment of- Skin and soft tissue infections: boils, abscesses, carbuncles, furunculosis, cellulitis; infected skin conditions, e.g. ulcer, eczema and acne; infected wounds, infected burns, protection for skin grafts, otitis media and externa, impetigo. Respiratory tract infections: pneumonia, lung abscess, empysema, sinusitis, pharyngitis, tonsillitis, and quinsy. Other infections caused by Flucloxacillin-sensitive organisms: osteomyelitis, enteritis, endocarditis, urinary tract infections, meningitis, septicaemia. Flucloxacillin is also indicated for use as a prophylactic agent during major surgical procedures where appropriate; for example, cardiothoracic and orthopaedic surgery. Dosage and directions for use: Oral doses should be administered half to one hour before meals. Usual adult dosage (including elderly patients): 250 mg four times daily. In severe infections, dosage should be doubled. In osteomyelitis and endocarditis: up to 8g daily, in divided doses 6 to 8 hourly. In case of secondary bacterial infection in chicken pox: Flucloxacillin 500 mg six hourly should be prescribed. Usual children dosage: 2-10 years: half of the adult dose. Under 2 years: quarter of the adult dose. Directions for Reconstitution Suspension: To prepare suspension, add sufficient quantity boiled & cooled water, mentioned in the individual product’s label. Add the total water required in two portions. Shake the bottle well in each addition until all the powder is in suspension. Shake the bottle well before use suspension. A cup is supplied to aid water measuring and corrects dosing of suspension. Use within 7 days after reconstitution. Side Effects: Generally well tolerated. Gastrointestinal upsets e.g. nausea, diarrhoea and skin rashes have been reported. If skin rash occurs, treatment should be discontinued. Precautions: Older patients receiving Flucloxacillin for longer than two weeks are at a risk of jaundice. In the presence of severe renal failure (creatinine clearance < 10ml/min) a reduction in dosage or an extension of dose interval should be considered. Contraindications: Flucloxacillin should not be given to patients with a history of hypersensitivity to β-lactam antibiotics (e.g. penicillins, cephalosporins) or excipients. Flucloxacillin is contra-indicated in patients with a previous history of flucloxacillin associated jaundice/hepatic dysfunction. Drug Interaction: Concurrent administration of probenicid delays the renal excretion of flucloxacillin. Since bacteriostatic agents (Chloramphenicol, Erythromycin, Sulfonamides or Tetracyclines) may interfere with the bactericidal effect of penicillins in the treatment of meningitis or other situations where a rapid bactericidal effect is necessary, it is best to avoid concurrent therapy. Use in Pregnancy & Lactation: Pregnancy Category B. There are no adequate and well-controlled studies in pregnant women. So this drug should be used during pregnancy only if clearly needed. It is not known whether Flucloxacillin is excreted in human milk. So it is probably best either to avoid using the drug by nursing mother or to discontinue breast feeding. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Surgeflox® 250 Capsule: Each box contains 5x4’s capsules in Alu-Alu blister pack. Surgeflox® 500 Capsule: Each box contains 4x10’s capsules in Alu-Alu blister pack. Surgeflox® PFS: Each bottle contains dry powder for 100ml oral suspension with a measuring cup & dropper.
-
Timopa®
Presentation: Timopa® Tablet: Each film coated tablet contains Tiemonium Methylsulfate INN 50 mg. Pharmacological action: Tiemonium Methylsulphate is an antispasmodic drug. Tiemonium strengthens calcium bonding with phospholipids and proteins thus stabilizing the cell membrane of the GI tract. It reduces the muscle spasm of the intestine, biliary system, bladder and uterus. Indications: Tiemonium Methylsulphate is indicated for the treatment of gastroenteritis, diarrhea, dysentery, biliary colic, enterocolitis, cholecystitis, colonopathies, mild cystitis, & spasmodic dysmenorrhoea. Dosage and directions for use: The recommended adult dose of Tiemonium Methylsulphate is 2-6 tablets (100-300 mg) daily in divided dosage as required or as directed by the physicians. Safety and effectiveness of Tiemonium Methylsulphate in paediatric patients have not been established. Side Effects: Side effect with the use of Tiemonium Methylsulphate is very rare. Some of the side-effects may be rare but serious such as tachycardia, hypotension. Precautions: Caution should be taken in cases of chronic bronchitis, coronary insufficiency, ambient hyperthermia, renal insufficiency, hepatic insufficiency, pregnancy and lactation. Contraindications: Tiemonium Methylsulphate should not be used in glaucoma or where acute pain of eyeball with vision disturbance was found. Also this drug should not be used in patients with the disorder of prostate or urinary bladder. Drug Interaction: Tiemonium Methylsulphate should not be used with other drugs without prior consult of a registered physician to avoid possible drug interaction. Use in Pregnancy & Lactation: Tiemonium Methylsulphate may be used in pregnancy & lactation only if it is clearly needed by the assessment of risk benefit ratio. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Timopa® Tablet: Each box contains 5x10’s tablets in blister pack.
-
Vecarot®
Presentation: Vecarot® Tablet: Each film coated tablet contains Beta carotene USP 6 mg, Ascorbic Acid BP 200 mg and Vitamin E BP 50 mg as DL-alpha-Tocopherol acetate. Pharmacological action: Beta-carotene is an essential vitamin needed for growth and development, cell recognition, vision, immune function, and reproduction. It is a powerful antioxidant and acts as a hormone in the body, affecting the expression of genes and thereby influencing phenotype. It also helps the heart, lungs, kidneys, and other organs to function correctly. Vitamin C is important in the defense against infection and studies shown that vitamin C is important for the normal functioning of T-lymphocyte and leukocyte. Ascorbic acid has some anti-inflammatory activity and protects cells against oxidation of essential molecules. Vitamin E acts as an antioxidant (i.e., an inhibitor of oxidation processes) in body tissues. It protects unsaturated fats in the body from oxidation by peroxides and other free radicals. Indications: This medication is a multivitamin product used to treat or prevent vitamin deficiency due to poor diet, certain illnesses, or as antioxidant. Vitamins are important building blocks of the body and help keep you in good health. Dosage and directions for use: One tablet daily or advised by the physician. Side Effects: Constipation, diarrhea, or upset stomach may occur. These effects are usually temporary and may disappear as your body adjusts to this medication. Precautions: A very serious allergic reaction to this drug is rare. However, seek immediate medical attention if you notice any of the following symptoms of a serious allergic reaction: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing. Contraindications: Contraindicated in patients with a known hypersensitivity to its components. Excessive doses of Vitamin A should be avoided in pregnancy because of potential teratogenic effects. Drug Interaction: There is no potentially hazardous drug interaction with retinol. Both cadmium and copper decreases retinol plasma levels. Among antibiotics neomycin and bleomycin reduces the absorption of retinol. Vitamin C is incompatible in solution with aminophylline, leomycin, erythromycin, lactobionate, nafcillin, nitrofurantoin , conjugated oestrogens, sodium bicarbonate, sulphafurazole, chloramphenicol sodium succinate, chlorothiazide sodium and hydrocortisone sodium succinate. It increases the apparent half-life of paracetamol and enhances iron absorption from the gastrointestinal tract. No potentially useful drug interaction with Vitamin E has been described. However, high doses of Vitamin E can impair intestinal absorption of Vitamin A and K. Use in Pregnancy & Lactation: This drug should be used during pregnancy only if clearly needed. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Vecarot® Tablet:Each HDPE container contains 30’s tablets.
-
Versal®
Presentation:
Versal® 20 Tablet: Each enteric coated tablet contains Esomeprazole 20mg as Esomeprazole Magnesium Trihydrate USP.
Versal® 20 Capsule: Each capsule contains Esomeprazole 20mg as enteric coated pellets of Esomeprazole Magnesium Trihydrate USP.
Pharmacological action: Esomeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+/K+-ATPase in the gastric parietal cell. By acting specifically on the proton pump, which blocks the final step in acid production, thus reducing gastric acidity.
Indications: The FDA approved Esomeprazole is indicated for Gastroesophageal Reflux Disease (GERD),Healing of Erosive Esophagitis, Maintenance of Healing of Erosive Esophagitis, Symptomatic Gastroesophageal Reflux Disease, H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence, Zollinger-Ellison Syndrome, Acid Related Dyspepsia Duodenal and Gastric Ulcer.
Dosage and directions for use: The usual recommended dose is 20 or 40 mg tablet/capsule once daily.
* The majority of patients are healed within 4 to 8 weeks. For patients who do not heal after 4-8 weeks, an additional 4-8 weeks treatment may be considered.
** Controlled studies did not extend beyond six months.
*** If symptoms do not resolve completely after 4 weeks, an additional 4 weeks of treatment may be considered.
Children:
FDA approved Esomeprazole for children aged 1-11 years. Dose: <20kg body weight 5 to 10mg/day and >20kg body weight 10 to 20mg/day.
Elderly: Dose adjustment is not required in the elderly.
Side Effects: The following adverse drug reactions have identified or suspected in the clinical trials programme for Esomeprazole. More frequent: Headache, abdominal pain, diarrhoea, flatulence, nausea/ vomiting and Less frequent: Dermatitis, pruritus, urticaria, dizziness.
Precautions: Symptomatic response to therapy with Esomeprazole does not preclude the presence of gastric malignancy. Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole, of which Esomeprazole is an enantiomer.
Contraindications: Known hypersensitivity to Esomeprazole, substituted Benzimidazoles or any other constituents of the formulation.
Drug Interaction: In common with the use of other inhibitors of acid secretion or antacids, the absorption of Ketoconazole and Itraconazole can decrease during treatment with Esomeprazole. When Esomeprazole is combined with drugs, such as Diazepam, Citalopram, Imipramine, Clomipramine, Phenytoin etc., the plasma concentrations of these drugs may be increased and a dose reduction could be needed.
Use in Pregnancy & Lactation: There are no adequate and well-controlled studies on the use of Esomeprazole in pregnant women. Therapeutic doses during pregnancy are unlikely to pose a substantial teratogenic risk. Esomeprazole should be used during pregnancy only if the potential benefit to pregnant women justifies the potential risk to the fetus. Esomeprazole is excreted in human milk. Thus, a decision should be taken to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Versal® 20 Tablet: Each box contains 5 x 10’s tablets in Alu-Alu blister pack.
Versal® 20 Capsule: Each box contains 5 x 10’s capsules in Alu-Alu blister pack.

-
Xpotil®
Presentation: Xpotil® PFS: After reconstitution, each 5 ml suspension contains Cefpodoxime 40mg as Cefpodoxime Proxetil USP. Pharmacological action: Cefpodoxime is a semisynthetic & third generation cephalosporin antibiotic with broad spectrum bactericidal activity against both gram-positive and gram-negative bacteria. It interferes with the synthesis of bacterial cell wall by inhibiting transpeptidase enzyme. As a result the bacterial cell wall is weakened, the cell swells and then ruptures. It is also highly active against most strains of penicillinase producing staphylococci. Indications: Cefpodoxime is used in the treatment of infections caused by sensitive organisms-- Upper respiratory tract infections.
- Lower respiratory tract infections.
- Urinary tract infections including gonorrhea.
- Skin and soft tissue infections.
- Gynaecological infections.
- Acute otitis media.
- Childhood infection.
- Typhoid fever of children.

-
Zeenk®
Presentation: Zeenk® Syrup: Each 5 ml syrup contains 10 mg elemental Zinc as Zinc Sulfate Monohydrate USP. Pharmacological action: Zinc is an essential trace element and involved in a number of body enzyme systems. Zinc salts are used as supplements to correct Zinc deficiencies. The consequences of Zinc deficiency are malformations of the brain, eyes, bones, heart and other organs. Zinc is essential for the formation and function of the immune system. Systems influenced by Zinc include the reproductive, neurologic, immune, dermatologic and gastrointestinal systems. Indications: Zinc is indicated for the following indications-Diarrhea, Prevention and treatment of colds, Zinc deficiency, Immune deficiency, Cancer prevention, Maintenance of taste and smell, Wound healing, Anemia, Night blindness, Mental disturbances, Severe growth retardation. Dosage and directions for use: The recommended dose in Children under 10 kg: 2 tea-spoonful daily in divided doses; Children within 10-30 kg: 2 tea-spoonful 1-3 times daily; Adults & Children over 30 kg: 4 tea-spoonful 1-3 times daily after food, or as directed by the physicians. Side Effects: Zinc salts may cause abdominal pain and dyspepsia. Prolonged use may lead to copper deficiency and anaemia. Precautions: Concurrent administration of Zinc salt with penicillamine might diminish the effect of Penicillamine. The absorption of Zinc, although poor, may be decreased by various compounds including some foods. Chelation may occur with tetracyclines. Contraindications: It is contraindicated in patients with hypersensitivity to Zinc. Drug Interaction: Zinc may inhibit the absorption of concurrently administered Tetracyclines, therefore, an interval of at least 3 hours should be allowed while taking these products. Use in Pregnancy & Lactation: Pregnant women and nursing mothers should avoid zinc doses higher than RDA amounts. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Pregnant women and nursing mothers should avoid zinc doses higher than RDA amounts. Zeenk® Syrup: Each bottle contains 100 ml syrup with a measuring spoon.
-
Zeeplex®
Presentation:Zeeplex® Syrup: Each 5 ml syrup contains Thiamine HCl BP 5 mg, Riboflavin 2 mg as Riboflavin 5-phosphate Sodium BP, Pyridoxine HCl BP 2 mg, Nicotinamide BP 20 mg, Elemental Zinc 10 mg as Zinc Sulphate Monohydrate USP.
Pharmacological action: B-Vitamins which compensates vitamin deficiency & promotes appropriate growth of the body and helps to increase body resistance against disease. The members of the vitamin B group are components of enzyme systems that regulate various stages of carbohydrate, fat and protein metabolism, each of the components playing a specific biological role. Zinc sulfate is a source of zinc which is an essential trace element required for human nutrition and involved in a number of body enzyme system.Indications: This preparation is indicated in treatment and prevention of B-vitamins and Zinc deficiencies.
Dosage and directions for use: The recommended dose in- Adult: 10 ml (2 teaspoon) 2 to 3 times daily or as recommended by the physician. Children: 10 ml (2 teaspoon) 1 to 3 times daily or as recommended by the physician. Infants: 5 ml (1teaspoon) 1 to 2 times daily or as recommended by the physician.
Side Effects: Generally well tolerated. However zinc salts may cause abdominal pain and dyspepsia.
Precautions: Concurrent administration of zinc salt with penicillamine might diminish the effect of penicillamine. The absorption of Zinc, although poor, may be decreased by various compounds including some foods. Chelation may occur with tetracyclines.
Contraindications: Contraindicated in case of hypersensitivity to any of the active ingredients.
Drug Interaction: Generally no interactions have been observed. Therefore, it is not recommended for patients undergoing such therapy.
Use in Pregnancy & Lactation: It is safe to use in pregnancy and lactation.
Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children.
Commercial Packaging:
Zeeplex® Syrup: Each bottle contains 100 ml & 200ml syrup with a measuring spoon.

-
Zenaf®
Presentation: Zenaf® 250 Capsule: Each capsule contains Cephradine USP 250mg. Zenaf® 500 Capsule: Each capsule contains Cephradine USP 500mg. Zenaf® PFS: After reconstitution, each 5 ml suspension contains Cephradine Anhydrous 125mg as Cephradine USP. Zenaf® PD: After reconstitution, each 1.25 ml Paediatric drop contains Cephradine USP 125mg. Pharmacological action: Cephradine is a semisynthetic & first generation cephalosporin antibiotic with broad spectrum bactericidal activity against both gram-positive and gram-negative bacteria. It interferes with the synthesis of bacterial cell wall by inhibiting transpeptidase enzyme. As a result the bacterial cell wall is weakened, the cell swells and then ruptures. It is also highly active against most strains of penicillinase producing staphylococci. It has a high degree of stability to many beta-lactamases. Indications: Cephradine is used in the treatment of infections caused by sensitive organisms.- Upper respiratory tract infections: Pharyngitis, sinusitis, otitis media, tonsillitis, laryngotracheo-bronchitis.
- Lower respiratory tract infections: Acute and chronic bronchitis, lobar and bronchopneumonia.
- Urinary tract infections: Cystitis, urethritis, pyelonephritis.
- Skin and soft tissue infections: Abscess, cellulitis, furunculosis, impetigo.
- Gastrointestinal tract infections: Bacillary dysentery, enteritis, peritonitis.
- Bone & joint infection.

-
Ziron-F®
Presentation: Ziron-F® Capsule: Each timed release capsule contains Dried Ferrous Sulphate BP 150 mg, Folic Acid BP 0.5mg and Zinc Sulphate Monohydrate USP 61.80 mg. Pharmacological action: Ferrous sulfate is a type of iron which is a part of hemoglobin and myoglobin. Folic Acid helps your body produce and maintain new cells, and also helps prevent changes to DNA that may lead to cancer. Folic acid is a B vitamin which is vital for the formation of red blood cells. Zinc acts as a cofactor for more than 70 different enzymes. It provides normal growth and tissue repair. It also helps in development of cell mediated immunity. Indications: Ziron-F® is indicated for the treatment and prophylaxis of iron, folic acid and zinc deficiency especially during pregnancy and lactation. Dosage and directions for use: Adults & Elderly: 1 capsule daily or as directed by the physician. In more severe cases, 2 capsules daily may be required. Children (Aged over 1 year): 1 capsule daily. The capsule may be opened and the pellets to be mixed with soft, cool food, but they must not be chewed. Side Effects: Side effects of iron, folic acid and zinc supplementation are mild and transient. These include epigastric pain, nausea, constipation, vomiting, diarrhoea, heart burn, etc. Allergic sensitization has been reported following oral administration of folic acid. Precautions: Care should be taken in patients who may develop iron overload, such as those with haemolytic anaemia, haemochromatosis or red cell aplasia. Failure to respond to treatment may indicate other causes of anaemia and should be further investigated. Contraindications: Do not use in patients hypersensitive to the components of the product or those with iron overload. Drug Interaction: Iron chelates with tetracycline. Since oral iron products interfere with absorption of oral tetracycline antibiotics, this product should not be taken within two hours of each other. Occasional gastrointestinal discomfort may be minimized by taking with meals. Absorption of iron may be impaired by concurrent administrations of penicillamine and antacid. In patients with renal failure, a risk of zinc accumulation may exist. Use in Pregnancy & Lactation: If pregnant, or planning to become pregnant or are currently breast-feeding please contact your physician, before taking or continuing the drug. Administration in first trimester of pregnancy should be avoided unless definite evidence of iron deficiency is observed. Prophylaxis of iron deficiency is justified during the remainder of pregnancy specifically when zinc supplementation is required. Pharmaceutical Precaution: Store in a cool and dry place, protected from light. Keep out of the reach of children. Commercial Packaging: Ziron-F® Capsule: Each box contains 5x10’s Timed Release capsules in blister pack.